1201
3D Diffusion Imaging with SPiral Encoded Navigators from Stimulated Echoes (3D DISPENSE)1Department of Radiology, Academical Medical Center, Amsterdam, Netherlands, 2Department of Biomedical Engineering and Physics, Academic Medical Center, Amsterdam, Netherlands
Synopsis
In this work, we present a new method for motion-insensitive 3D multi-shot diffusion imaging, by using 3D Diffusion Imaging with SPiral Encoded Navigators from Stimulated Echoes (3D DISPENSE). The 3D spiral navigator is acquired with a single readout and generated between diffusion preparation and image acquisition from the twin-pathway of the stimulated echo TSE imaging signal pathway. Therefore, the proposed 3D navigator technique does not compromise diffusion weighting or TSE readout efficiency. We demonstrated the feasibility of this method in phantoms, as well as by in vivo 3D high resolution artifact-free diffusion tensor imaging with nearly full brain coverage.
Introduction
Multi-shot sequences are widely used for high-resolution diffusion-weighted imaging (DWI),1-3 but this approach suffers from inter-shot phase incoherence due to subtle motion, leading to severe image blurring and signal voids.4-6 To fully calibrate the phase incoherence among different shots, an image navigator with the same dimension (2D or 3D) for each shot is needed. For the 2D case, the relatively short 2D navigator signal may be acquired at the start or end of imaging readout. However, this is highly challenging for the 3D case as T2 signal decay during readout will compromise either the 3D imaging or 3D navigator signal-to-noise ratio (SNR).
In this work, we propose an extension to the 3D diffusion prepared stimulated echo TSE sequence (DPsti-TSE),7 in which a 3D spiral navigator signal can be generated almost for free from the twin signal pathway of the stimulated imaging echo in between diffusion preparation and imaging readout. This twin-pathway contains the same phase information as the imaging echo and therefore can be used to correct multi-shot phase incoherence. The proposed sequence is demonstrated using phantom experiments and applied for in vivo brain diffusion tensor imaging (DTI).
Methods
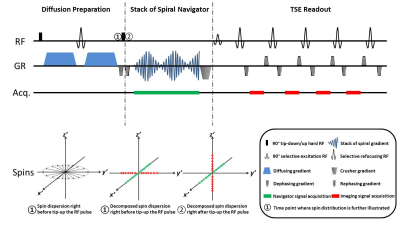
Sequence: The basic DPsti-TSE sequence (Fig.1) features a dephasing gradient at the end of the diffusion preparation module to eliminate the influence of the phase offset and dispersion caused by eddy currents.7 After the 90° tip-up RF pulse, half of the diffusion prepared magnetization is stored in the longitudinal direction and then forms the imaging signal in the TSE module following a stimulated echo pathway (Fig.1, red). The other half of the magnetization is left in the transverse plane, which now is used to generate the 3D spiral encoded navigator signal (Fig.1, green).
3D spiral navigator: The navigator signal is acquired in a single readout window, during which no RF pulse is applied to avoid any interference with the subsequent TSE imaging readout. A cylindrical k-space trajectory with constant spiral density is used. Shot-by-shot low resolution navigator images are then reconstructed using a non-uniform Fourier transform method,8 and interpolated to the same resolution as the 3D TSE images.
TSE image reconstruction: Diffusion weighted TSE images are reconstructed by minimizing the L2-norm regularized energy function below, using a conjugate gradient (CG) algorithm. $$\min_{I\in{\mathbb{C}}}\sum_{c,k}||m_{c,k}-FS_cP_kI||_2^2+\lambda||I||_2^2$$ where $$$I$$$ is the desired artifact free diffusion weighted image; $$$P$$$ is the phase error estimated from 3D navigator; $$$S$$$ is the sensitivity encoding; $$$F$$$ is the Fourier transform; $$$m$$$ is the measured TSE image k-space signal; $$$\lambda$$$ is the regularization parameter.
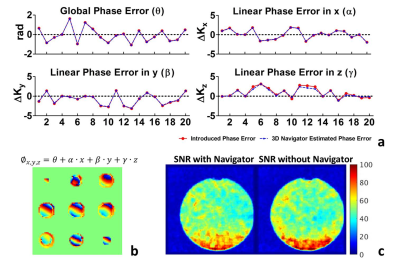
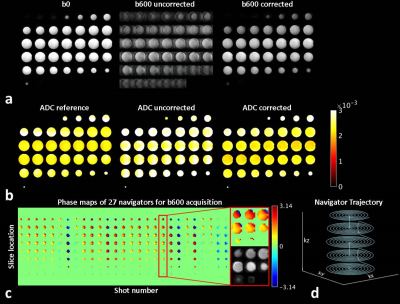
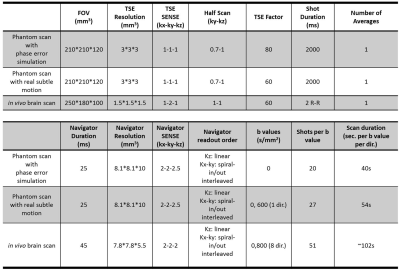
Phantom measurements: A phantom (T1: 1137ms; T2: 900ms; T2*: 103ms) was scanned on a Philips Ingenia 3T scanner. To validate the performance of the 3D navigator, known linear and global phase errors were deliberately introduced by adding gradients in the diffusion module and compared to the estimations from the navigator. To investigate the potential TSE image SNR loss, SNR maps were calculated for phantom TSE images acquired with and without the presence of a 100ms navigator. Furthermore, the phantom was scanned during the course of a subtle motion introduced by pulling the phantom gently back and forth in the scanner using a rope. Navigator corrected images and apparent diffusion coefficient (ADC) maps were compared with uncorrected and motion-free reference images.
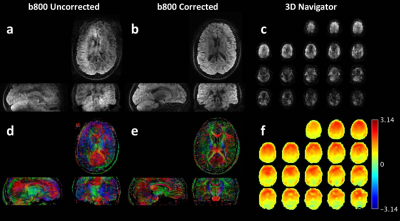
In vivo measurement: A brain DTI scan was performed on a healthy volunteer (26, Female) with cardiac synchronization. Colored FA maps derived from navigator corrected and uncorrected images were compared. Detailed scanning parameters are listed in Table 1. Data was processed and visualized using MATLAB (The Mathworks, Natick MA).
Results & Discussion
Fig.2a shows that the estimated phase error parameters matched well with the known phase errors, with a global and linear coefficient estimation error mean and standard deviation (SD) of 0.02±0.02rad and 0.11±0.12Δk , respectively. When using 3D navigator, the mean SNR dropped from 49.1 to 43.2 due to some additional T1 decay of the stimulated echo signal.
Fig.3a illustrates the phantom reconstruction results in the presence of deliberately introduced subtle motion. The artifact can be greatly mitigated with the proposed 3D spiral navigator correction algorithm. Fig.3b illustrates that the ADC estimation after correction was identical to the reference (mean and SD = 2.4±0.3mm2/s), while the uncorrected ADC was 2.8±0.4mm2/s.
Fig.4 shows the in vivo brain DTI results. Artifact were removed almost perfectly in all view angles by the proposed method. As a result, colored FA maps were improved dramatically.
Conclusion
In this study, we showed the feasibility of performing motion-insensitive multi-shot diffusion imaging using 3D stack-of-spiral navigators. This method will have great potential for 3D isotropic high-resolution diffusion imaging.Acknowledgements
No acknowledgement found.References
- O’Halloran RL, Aksoy M, Van a. T, Bammer R. 3D isotropic high-resolution diffusion-weighted MRI of the whole brain with a motion-corrected steady-state free precession sequence. Magn Reson Med. 2013;70:466-478. doi:10.1002/mrm.24489.
- Liu C, Bammer R, Kim D-H, Moseley ME. Self-navigated interleaved spiral (SNAILS): application to high-resolution diffusion tensor imaging. Magn Reson Med. 2004;52(6):1388-1396. doi:10.1002/mrm.20288.
- Truong TK, Guidon A. High-Resolution Multishot Spiral Diffusion Tensor Imaging with Inherent Correction of Motion-Induced Phase Errors. Magn Reson Med. 2014;71(2):790-796. doi:Doi 10.1002/Mrm.24709.
- Miller KL, Pauly JM. Nonlinear phase correction for navigated diffusion imaging. Magn Reson Med. 2003;50(2):343-353. doi:10.1002/mrm.10531.
- Liu C, Moseley ME, Bammer R. Simultaneous phase correction and SENSE reconstruction for navigated multi-shot DWI with non-cartesian k-space sampling. Magn Reson Med. 2005;54(6):1412-1422. doi:10.1002/mrm.20706.
- Chu ML, Chang HC, Chung HW, Truong TK, Bashir MR, Chen NK. POCS-based reconstruction of multiplexed sensitivity encoded MRI (POCSMUSE): A general algorithm for reducing motion-related artifacts. Magn Reson Med. 2015;74(5):1336-1348. doi:10.1002/mrm.25527.
- Zhang Q, Coolen BF, Versluis MJ, Strijkers GJ, Nederveen AJ. Diffusion-prepared stimulated-echo turbo spin echo (DPsti-TSE): An eddy current-insensitive sequence for three-dimensional high-resolution and undistorted diffusion-weighted imaging. NMR Biomed. 2017;30(7):1-12. doi:10.1002/nbm.3719.
- Fessler JA, Member S, Sutton BP. Nonuniform Fast Fourier Transforms Using Min-Max Interpolation. 2003;51(2):560-574.
Figures