1200
Multi-contrast EPI - Towards clinical application1MR Applied Science Laboratory Europe, GE Healthcare, Stockholm, Sweden, 2Karolinska University Hospital, Dept of Neuroradiology, Stockholm, Sweden, 3Karolinska Institute, Dept of Clinical Neuroscience, Stockholm, Sweden
Synopsis
We present an enhanced version of our multi-contrast EPI sequence which generates T1-FLAIR, T2-FLAIR, T2*w, T2w, iso-DWI, ADC images of 36 slices in 90 s. We introduce various optimization including tetrahedral diffusion encoding, different partial Fourier factors for the EPI trains and multi-echo acquisition for T2-FLAIR, T2*w, T2w, and DWI. The improved sequence is compared to our previous implementation and superior image quality is shown especially for the diffusion contrast. Finally, multi-contrast EPI patient data is shown and compared to conventional imaging sequences.
Purpose
MRI screening in neuroimaging for patients with unclear symptoms involves several contrasts usually exceeding 10 minutes of scan time. Moreover, for acute cases, CT is preferred over MRI, despite its inferior soft tissue contrast. We have recently presented a new multi-contrast EPI [1] sequence for neuroimaging, which generates six MR contrasts (T1-FLAIR, T2-FLAIR, T2*w, T2w, iso-DWI, ADC) in about one minute with full brain coverage. These contrasts correspond well to the diagnostic requirements for brain trauma, edema, inflammation, stroke, contrast leaking tumors and bleeding. Due to its single shot nature, the multi-contrast EPI sequence is very robust against motion. This work focuses on increasing the image quality further, especially of the diffusion contrast to facilitate acute stroke diagnosis and of the T2-FLAIR contrast to improve the delineation of white matter lesions and brain inflammations.Methods
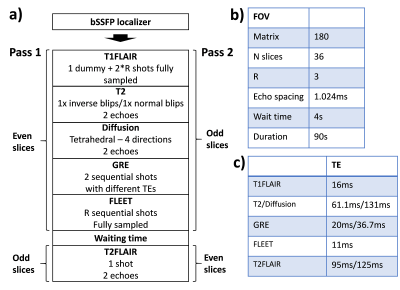
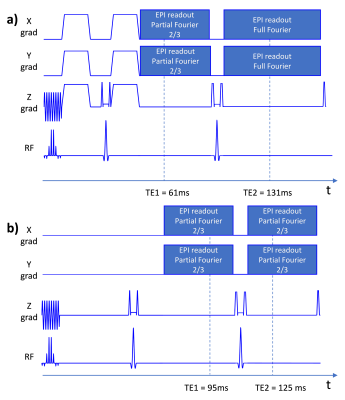
The sequence ordering is overviewed in Fig. 1a. The slices were divided in two passes (odd/even slices) to allow widening of the inversion slice (for T1-FLAIR and T2-FLAIR) without cross-talks. Widening the inversion slice reduces magnetization history effects and CSF inflow effects [1]. Relevant sequence parameters are summarized in Fig. 1b-c. To increase the SNR of the diffusion acquisition, partial Fourier of 2/3 and tetrahedral encoding was used to reduce TE to 61 ms compared to 86 ms in our previous implementation. This reduced TE makes however the b=0 volume less suitable as a clinical T2w contrast. Moreover, the TR of the diffusion acquisition would be below 2 s, leading to unwanted T1 saturation. Therefore, a second refocusing pulse was played out followed by a full Fourier EPI train, enabling a second diffusion echo (Fig. 2a), with stronger T2-weighting and also a longer diffusion TR of about 3 s. The T2-FLAIR acquisition was also composed of two echoes (EPI trains) both with partial Fourier of 2/3, straddling a second refocusing pulse (see Fig. 2b). Two separated GRE echoes were acquired as well, one with a shorter TE=20 ms and partial Fourier of 2/3 and one with a long TE=36 ms and full Fourier encoding. The two echoes involved for the T2w, DWI, T2-FLAIR and GRE (T2*w) contrasts were averaged. For comparison, we used our previous implementation of multi-contrast EPI [1] with an overall scan time of 80 s compared to 90 s of the new implementation. Motion and eddy current correction were performed using a 2D slice to slice affine registration of all image data to the T2w contrast [2]. Patients and healthy volunteers were scanned on a GE MR750w system (GE Healthcare, WI, USA) using an 8-ch RF receive coil.Results
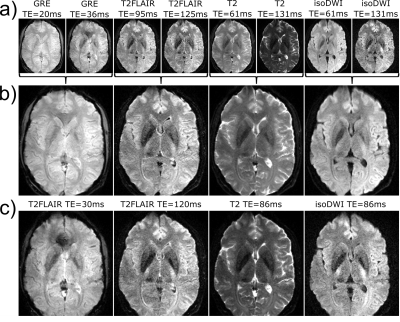
Fig. 3a shows the two echoes for the GRE, T2-FLAIR, T2w and DWI acquisition, respectively. Fig. 3b depicts the corresponding averaged contrasts and Fig. 3c shows the results from our previous implementation. There is a large SNR increase for the iso-DWI contrast and moderate increases in SNR for T2-FLAIR, GRE and T2w. Although the T2 and T2* weighting is slightly reduced in favour of a better image quality the late echoes (Fig. 3a) could be used as fallback if a stronger weighting is desired.
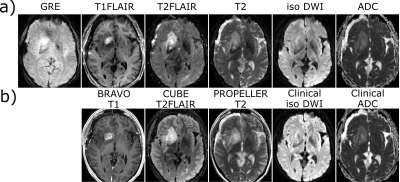
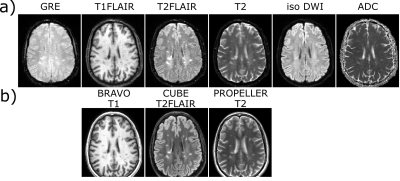
Comparisons of patient scans using multi-contrast EPI and conventional clinical MR images are shown in Fig. 4 and 5. Fig. 4 depicts images of a tumor patient post-Gd. Despite the lower resolution, there is an excellent agreement between multi-contrast EPI and the conventional sequences in terms of tumor delineation and gadolinium enhancement. The multi-contrast EPI T2-FLAIR suffers from some inflow artifacts especially outside the skull, which can also be easily identified in the iso-DWI image. Fig. 5 depicts images of an Multiple Sclerosis (MS) patient. The white matter lesions can be clearly identified in the T2-FLAIR and T2w contrasts.
Discussion
We have presented an improved version of our multi-contrast EPI sequence, with two EPI echoes for many of the contrasts and with tetrahedral diffusion encoding. The acquisition of multiple echoes increases the SNR efficiency and provides also flexibility in the way the echoes can be combined. All multi-contrast EPI contrasts are acquired in the same coordinate system, with identical geometrical distortions and similar point spread functions, which may be of particular interest for the present rise of machine learning techniques. Here, the additional information of multiple echoes may also be exploited to identify or classify pathologies. Overall, good agreement between the multi-contrast EPI and conventional image data was shown. Next, a larger study involving neuroradiologists will show the opportunities and limits of multi-contrast EPI in the clinical practice.Acknowledgements
No acknowledgement found.References
1. Skare, S. et al. A 1-minute full brain MR exam using a multi-contrast EPI sequence. Magn. Reson. Med. (2017). doi:10.1002/mrm.269742.
2. Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW. elastix: a toolbox for intensity-basedmedical image registration. IEEE Trans. Med. Imaging 2010;29:196–205.
Figures