1195
Long-Term Volumetric Thermometry for Measurement of Diffuse Tissue Heating during MRgFUS Treatments1Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States
Synopsis
Currently, PRF MR thermometry is commonly used for temperature monitoring and thermal dose estimation during MRgFUS treatments. The temperature is calculated based on the phase change between the current image and the baseline image, which is acquired before each sonication starts. This does not account for residual heat from previous sonications. In this work, we have developed a volumetric long-term thermometry technique to measure the change in temperature of the entire region of treatment for the duration of the procedure
Introduction
Currently, MR thermometry using the proton resonance frequency shift (PRF) is commonly used for temperature monitoring and thermal dose estimation during MRgFUS treatments. Clinical treatments of soft tissue tumors often consist of more than 100 short sonications (20-30 s each). The temperature is calculated based on the phase change between the current image and the baseline image, which is acquired before each sonication starts. However, since the next sonication often starts before the tissue has returned to body temperature, any residual heat within the tissue is not accounted for when the next baseline is taken. This has been shown to result in temperature and thermal dose underestimation within the tumor1.
Referenceless2 and hybrid3 techniques create temperature maps that accurately depict the small heating of individual sonications, but fail in this scenario of a large, diffuse heating area. Multi-baseline thermometry with drift correction4, proposed for hyperthermia treatments, could not provide flexibility in changing the treatment plan during the procedure.
The goal of this work was to develop a long-term thermometry technique to measure the change in temperature of the entire region of treatment for the duration of the procedure by implementing a short volumetric thermometry acquisition that can be run multiple times throughout the treatment and a post-processing technique to extract thermometry data in the presence of phase shift artifacts.
Methods
Volumetric thermometry was performed using two imaging protocols: 2D (FSPGR, TE=8-10 ms, TR=210 ms, 17-20 slices, FOV=280-320 mm, slice=6 mm, Tacq=30 sec) and 3D (FGRE, TE=6-8 ms, TR=11-16 ms, FOV=230-320 mm, slab=64-140 mm, Tacq=30 sec). Before each volumetric thermometry acquisition, the ultrasound transducer was moved to the home position to minimize phase artifacts due to metal elements.
Long-term thermometry is difficult to achieve with simple baseline subtraction. Factors such as field drift, susceptibility changes, and transducer or patient motion affect the image phase. Our temperature reconstruction method was based on a combination of phase subtraction with additional 3D phase corrections. First, to avoid phase wrapping after subtraction, a constant phase normalization was applied to all datasets. After baseline subtraction, linear phase correction was performed by fitting a first order polynomial in a 3D ROI of unheated tissue surrounding the treatment area or in subcutaneous fat.
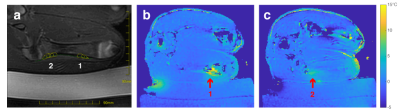
The acquisition protocols were tested in a tissue-mimicking phantom and in two pigs using an ExAblate 2100 MRgFUS system (InSightec, Israel) integrated with a 3T scanner (GE Healthcare, Waukesha, WI). In each animal, 12 sonications were performed in two locations on distal and proximal femur (Fig 1a). Volumetric thermometry images were acquired before the first sonication, after 6 sonications in the first location, and at the end of the treatment.
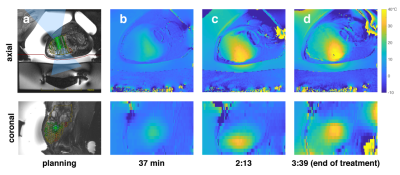
MRgFUS ablation was performed in one patient with a desmoid tumor in the lower extremity. The patient received 104 treatment sonications (Fig 2a, 19-36 sec, 50-144W) with 4 verification sonications over the course of around 4 hours. Volumetric thermometry images were acquired before the first sonication, after 14 and 60 sonications (37 and 133 min), and at the end of the treatment (219 min).
Results
The phantom experiment demonstrated the feasibility of volumetric thermometry with prior baseline after mechanical motion of the transducer. Temperature maps from the animal experiment showed residual heating from the first 6 sonications in the distal femur of 8°C (Fig 1b) and the remaining 6 sonications in the proximal femur of 5°C (Fig 1c).
Thermometry data from the desmoid tumor treatment (Fig 2) showed increasing diffuse temperature elevation within the tumor that followed recently treated areas and reached temperature of more than 30°C from baseline. The frequency shift correction algorithm reduced the temperature standard deviation in the subcutaneous lipid ROI from 9.07° to 3.85°C.
Discussion
Both 2D and 3D protocols produced similar temperature values, with 2D showing better SNR. Linear phase correction resulted in consistent thermal data for all time points with low temperature variation in adipose tissue and tissues outside the treatment area. This technique did not require phase unwrapping, which is complicated in 3D datasets with fatty and aqueous tissues.
The data from the patient treatment showed high temperature elevations in a large diffuse volume. More frequent measurements will help distinguish between residual heating from the last sonication and the accumulated heat from the earlier sonications.
Availability of spatial temperature data before each sonication will result in more precise estimation of the absolute temperature at the focal point, allowing to reduce the sonication energy. Measurement of accumulated heating from the focused ultrasound sonications will make more accurate calculation of thermal dose possible. This may allow for shorter, less costly MRgFUS treatments.
Acknowledgements
We would like to thank Radhika Tibrewala for help with proofreading the abstract.References
1. Bitton RR, Webb TD, Pauly KB, Ghanouni P. Improving thermal dose accuracy in magnetic resonance‐guided focused ultrasound surgery: Long‐term thermometry using a prior baseline as a reference. J Magn Reson Imaging. 2016;43(1):181-189. doi:10.1002/jmri.24978.
2. Rieke V, Vigen KK, Sommer G, Daniel BL, Pauly JM, Butts K. Referenceless PRF shift thermometry. Magnetic Resonance in Medicine. 2004;51(6):1223-1231. doi:10.1002/mrm.20090.
3. Grissom WA, Rieke V, Holbrook AB, et al. Hybrid referenceless and multibaseline subtraction MR thermometry for monitoring thermal therapies in moving organs. Medical Physics. 2010;37(9):5014-5026. doi:10.1118/1.3475943.
4. Tillander M, Hokland S, Koskela J, et al. High intensity focused ultrasound induced in vivo large volume hyperthermia under 3D MRI temperature control. Medical Physics. 2016;43(3):1539-1549. doi:10.1118/1.4942378.
Figures