1194
Intra-vascular, MRI-Guided, perivascular ultrasound ablation with thermometric monitoring of therapy delivery1Electrical and Computer Engineering, Johns Hopkins University, Baltimore, MD, United States, 2Russell H. Morgan Dept. of Radiology, Johns Hopkins University, Baltimore, MD, United States, 3Acertara Acoustic Laboratories, Longmont, CO, United States, 4Acoustic MedSystems, Inc, Champaign, IL, United States
Synopsis
Vessel invasion from a tumor is a major challenge for both surgical resection and extracorporeal HIFU ablation. Conceivably, an intravascular ultrasound ablation catheter combined with an intravascular high-resolution MRI coil could precisely target and monitor of peri-vascular therapy delivery that preserves the vessel wall via MRI thermometry. We present results from an intra-vascular 3T ultrasound ablation/MRI antenna probe that document MRI thermometry-based thermal dosage and lesion formation in porcine liver and chicken specimens with vessel wall preservation. A simple motion-correction method that employs the antenna’s intrinsic sensitivity profile as a navigator for thermometry is tested in vivo.
Purpose
The involvement of blood vessels with tumors is an important factor in the surgical management of tumor treatment. The extent of tumor-vessel contact and/or irregularity of the vessel contour can signify tumor involvement and vascular invasion(1). Unresectability aggravates the prognosis for locally-advanced pancreatic adenocarcinoma thus, potentially limiting treatment options. Extracorporeal high-intensity focused ultrasound (HIFU) ablation is an alternative to surgical resection, but requires precise focusing of the energy and tracking abdominal motion to achieve effective heating in the presence of air, vital organs and vessels in the beam’s pathway. Required MR system complexity for HIFU are also extensive. These factors and the need to avoid healthy tissue and vessels near the target itself remain challenging and a source of potentially significant complications(2).
The feasibility of guiding an intravascular ultrasound ablation catheter and monitoring therapy delivery via MRI thermometry performed with an intravascular MRI loopless antenna was recently demonstrated(3). However, the technology’s potential for ablating peri-vascular tissue while preserving immediate vital vessel wall guided by higher-resolution thermometry is yet to be demonstrated. Here we present results from a 3T ultrasound ablation/MRI antenna probe that document MRI thermometry-based thermal dosage and lesion formation, vessel wall preservation during peri-vascular procedures, and a simple motion-correction method for thermometry employing the antenna’s intrinsic sensitivity profile as a navigator.
Methods
Two types of intravascular ultrasound ablation catheters (flat 6.3 MHz transducer, 60° ablation angle; circular 7.4 MHz transducer, 360° ablation angle; both encapsulated in water-cooled jackets) from Acoustic MedSystems, Inc(4) were combined with an intravascular MRI loopless antenna(5,6,7), tuned and matched to a 3T Philips MRI system. MRI was performed with percutaneous ablation experiments in chicken muscle and intravascular ablation in pig liver specimens maintained at 32-37°C via a water bath in the scanner.
Upon catheter insertion, high-resolution MRI was performed to localize the transducers (bFFE, resolution 0.3mm), followed by a 5-min period during which cooling water was circulated in the probe to pre-cool the adjacent tissue/vessel wall. High resolution MRI thermometry(8) (proton resonant frequency shift; resolution 0.3mm; ~6s/scan) was used to monitor cooling and ultrasound ablation applied at 6.9W for 6-11min. Thermometry data was sent to a local computer (Philips XTC protocol)(9) for real-time temperature monitoring.
Tissues were subsequently dissected at ablation sites, photographed and manually delineated to correlate with thermal dose determined from MRI thermometry. The Jaccard index was used to assess the similarity of ablated regions of tissue identified by thermometry and histology. Movat staining was also performed to demarcate ablation zones from vessel wall.
A motion correction method was developed employing detection of the highly non-uniform intensity profile of the intravascular coil as an intrinsic navigator, and tested in vivo in a pig (1mm resolution; ~0.3s/scan).
Results
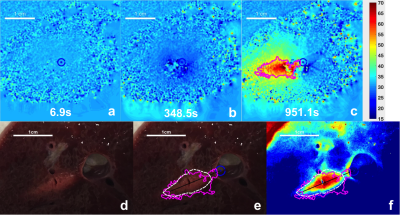
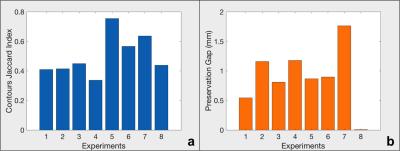
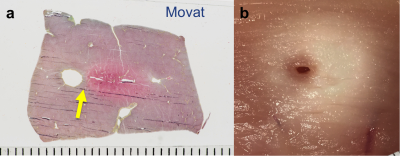
Real-time thermometry monitoring was available for every stage of the ablation operations: localization, cooling and ablation, as exemplified in a pig liver (Fig.1.a-c). The corresponding lesion is evident in a photo of the dissection at the ablation site (Fig1.d-f) with the 240 EM (cumulative equivalent minutes)(10) thermal dose contour calculated from the MRI thermometry (Fig.2.c) overlaid. Results from 8 consecutive perivascular ablation experiments on pig livers analyzed with the Jaccard index for characterizing similarities between thermometry and histology are shown in (Fig.3a), and the gaps between the vessel wall and the lesion are presented in (Fig.3b). Movat-stained histology of a pig liver ablation shown in (Fig.4a) and a photo of chicken muscle ablated with the circular transducer (Fig.4.b) both illustrate preservation of tissue immediately adjacent to the catheter/vessel. Typical preservation radial distances of 1-2 mm are readily achievable.
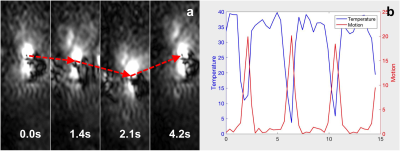
In vivo, transverse magnitude profiles of the loopless antenna show its suitability for motion tracking as a navigator to correct thermometry (Fig.5a,b).
Discussion
This study demonstrates the ability to reproducibly acquire high-resolution intra-vascular MRI thermometry for targeting and monitoring peri-vascular intravascular ultrasound ablation. Via comparison to gross histology, the results demonstrate that the intravascular thermometry results are sufficient to predict the spatial extent of lesion formation. This method could be used to titrate ultrasound power to control lesion size and extent, based upon acoustic power and treatment duration. However, further studies will be needed to evaluate delivery in vivo, which may be affected by differences in acoustic coupling and dominantly, perfusion. Importantly, the work shows that vessel wall preservation during peri-vascular ablation is reproducibly achievable, and this may be aided in vivo by blood flow that was not present in the current studies. Finally we observe that the effect of motion in vivo is potentially addressable by using the antenna’s sensitivity profile as a navigator in the image field-of-view.Acknowledgements
Grant support: NIH R01 EB007829.References
1. Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, Macari M, Megibow AJ, Miller FH, Mortele KJ, Merchant NB, Minter RM, Tamm EP, Sahani DV, Simeone DM. Pancreatic Ductal Adenocarcinoma Radiology Reporting Template: Consensus Statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology. 2014;270(1): 248-260
2. Khokhlova TD and Hwang JH. HIFU for palliative treatment of pancreatic cancer. Gastrointest Oncol. 2011;2:175-184
3. Liu X, Ellens N, Williams E, Burdette EC, Karmarkar P, Bottomley PA. Proc. Intl. Soc. Mag. Reson. Med. 25. 2017;1178
4. Ellens N, Partanen A, Ghoshal G, Burdette E and Farahani K. Integration of Interstitial High Intensity Therapeutic Ultrasound Applicators On a Clinical MRI-Guided High Intensity Focused Ultrasound Treatment Planning Software Platform. Med. Phys. 2015;42:3264.
5. El-Sharkawy AMM, Qian D, and Bottomley PA. The performance of interventional loopless MRI antennae at higher magnetic field strengths. Med. Phys. 2008;35(5):1995-2006.
6. Sathyanarayana S and Bottomley PA. MRI endoscopy using intrinsically localized probes. Med. Phys. 2009;36(3):908-919.
7. Sathyanarayana S, Schär M, Kraitchman DL, and Bottomley PA. Towards Real-Time Intravascular Endoscopic Magnetic Resonance Imaging. JACC: Cardiovascular Imaging. 2010;3(11):1158-1165.
8. Rieke V, and Pauly KB. MR Thermometry. J Magn Reson Imaging. 2008;27(2):376–390.
9. Zaporzan B, Waspe AC, Looi T, Mougenot C, Partanen A, Pichardo S. MatMRI and MatHIFU: software toolboxes for real-time monitoring and control of MR-guided HIFU. Journal of Therapeutic Ultrasound. 2013;1:7
10. McDannold NJ, King RL, Jolesz FA, and Hynynen KH. Usefulness of MR Imaging–Derived Thermometry and Dosimetry in Determining the Threshold for Tissue Damage Induced by Thermal Surgery in Rabbits. Radiology. 2000;216:517-523.
Figures