1193
Correction of focused ultrasound beam defocusing in heterogeneous soft tissues1Radiology and Imaging Sciences, University of Utah, Salt Lake City, UT, United States, 2Bioengineering, University of Utah, Salt Lake City, UT, United States, 3Electrical and Computer Engineering, University of Utah, Salt Lake City, UT, United States
Synopsis
Soft tissue MRgFUS treatments can be adversely affected by aberration of the focused ultrasound beam due to speed of sound differences between heterogeneous tissues. A quasi-real time beam aberration correction technique that uses an MRI derived model is presented and experimentally validated in a heterogeneous breast-mimicking phantom model. Comparison of MRgFUS sonications performed with and without phase aberration correction demonstrates that this model-based correction algorithm results in improved MRgFUS treatment efficiency and accuracy. This is shown to affect both thermal and mechanical MRgFUS applications.
Introduction
Ultrasound beam aberration correction is required in transcranical magnetic resonance guided focused ultrasound (MRgFUS) procedures due to the high acoustic impedance mismatch between the skull and soft tissues. While preliminary studies indicate that beam aberration correction can also improve the efficiency and accuracy of focused ultrasound in heterogeneous soft tissue applications1-3, it has not been applied clinically. Of several beam correction techniques proposed4-6, model-based, non-iterative methods have been shown to significantly reduce aberration losses in simulation and experimental studies in transcranial applications. This work applies this technique in a tissue-mimicking heterogeneous breast phantom to demonstrate the potential utility of model-based phase aberration correction to improve MRgFUS treatment efficiency and accuracy in soft tissue applications.Methods
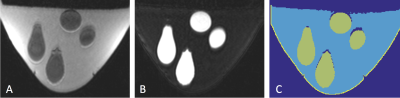
Phantom: A breast-mimicking phantom was constructed to replicate acoustic, thermal and mechanical properties of breast tissue. In a breast-shaped mold, fibroglandular tissue was modeled using gelatin and breast fat was mimicked by dispersing several small balloons (1-3 cm in diameter) filled with canola oil throughout the phantom. Acoustic properties of the gelatin and oil were measured using through-transmission and radiation force balance techniques2. A volumetric numerical model of the phantom (Figure 1) was created through automatically segmenting fat and water MR images using a 3D two-point Dixon GRE sequence (see Table 1).
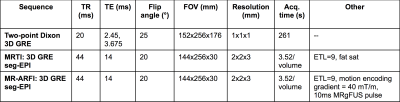
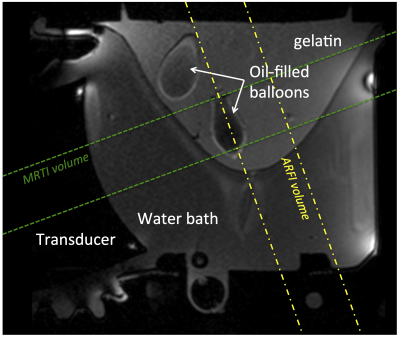
MRgFUS: Four points were sonicated in the phantom with position changes implemented using the multiple degrees-of-freedom in the breast-specific MRgFUS system7 (Figure 2). At each point, both MRgFUS (56W, 20s) and MR-Acoustic Radiation Force Imaging (ARFI) (56W, 10ms) sonications were performed with and without phase aberration correction and were monitored in real time with 3D MR temperature imaging (MRTI) and 3D simultaneous thermometry and ARFI imaging (see Table 1 for detailed parameters) sequences8. Relative to the focused ultrasound beam, the imaging slabs were oriented parallel for MRTI and perpendicular for MR-ARFI (Figure 2).
Phase aberration correction: The phase aberration correction algorithm9 applies the Hybrid Angular Spectrum (HAS) simulation method10 in quasi-real time to a 3D model of the anatomy, using accurate acoustic properties for all tissue types, and registration to the focused ultrasound transducer as inputs. The HAS algorithm rapidly calculates the 3D pressure pattern and computes the phase required at each transducer element to restore constructive interference at the focal location.
Comparison metrics: Technique effectiveness was determined by comparing the following metrics for the non-corrected and aberration-corrected acquired MRTI and MR-ARFI displacement maps: [1] the peak measured value, [2] the contiguous focal volume size at a threshold of 50% of the peak measured value, and [3] the distance to the intended location as determined through radiofrequency positioning coils integrated into the MRgFUS system11.
Results
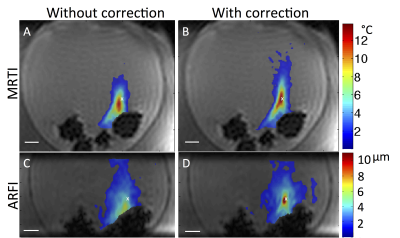
Phase aberration correction improved the efficiency and accuracy of the MRgFUS focus in both MRTI and MR-ARFI sonications in a clinically relevant time frame. After initial model generation (6 minutes), phase correction can be computed for each point in 1.5 minutes using a standard desktop computer. MR temperature and displacement maps, with and without phase aberration correction, for one of the four sonicated points are shown in Figure 3. In MRTI sonications (Fig. 3, A&B), phase aberration correction increased the maximum temperature (mean = +7%, range= +2.4% to +11.2%), decreased the focus volume indicating an increased concentration of thermal energy deposition (mean = -30.0%, range = -16.4 to -51.3%), and moved the focal point closer to the intended location (mean improvement = 2.0mm, range = -0.4 to 3.4mm). In the displacement measurements using MR-ARFI (Fig. 3, C&D), phase aberration correction increased the measured displacement (mean = +58.0%, range +4.0 to +135.5%), decreased the displacement volume (mean = -50.0%, range = -10.6 to -86.3%) and moved the focus closer to the intended location (mean improvement = 1.8mm, range = -2.37 to 6.33 mm).Discussion & Conclusions
This work has experimentally demonstrated the benefits of applying a phase aberration correction algorithm originally designed for transcranial MRgFUS, to soft tissue applications. Simulation studies have shown that the speed of sound difference between breast fat and fibroglandular tissue (only ~ 4%) can induce focused ultrasound beam aberrations that reduce treatment efficiency by decreasing energy deposition at the intended focal location and increasing near-field heat deposition2. The beam aberration correction technique presented here can be applied in a clinically relevant time frame to the very heterogeneous and patient-specific breast anatomy and yields improved beam focusing efficiency and accuracy in both temperature and displacement maps. The same method could be applied to other body regions where fat distributions are heterogeneous and patient specific.Acknowledgements
This work was funded by NIH grant R01 CA172787.References
1. Tabei M, Mast TD, Waag RC. Simulation of ultrasonic focus aberration and correction through human tissue. J Acoust Soc Am. 2003;113(2):1166-76.
2. Farrer AI, Almquist S, Dillon CR et al. Phase aberration simulation study of MRgFUS breast treatments. Med Phys. 2016;43(3):1374-84.
3. Mougenot C, Tillander M, Koskela J et al. High intensity focused ultrasound with large aperture transducers: a MRI based focal point correction for tissue heterogeneity. Med Phys. 2012;39(4):1936-45.
4. Hynynen K, Sun J. Trans-skull ultrasound therapy: the feasibility of using image-derived skull thickness information to correct the phase distortion. IEEE Trans Ultrason Ferroelectr Freq Control. 1999;46(3):752-5.
5. Vyas U, Kaye E, Pauly KB. Transcranial phase aberration correction using beam simulations and MR-ARFI. Med Phys. 2014;41(3):032901.
6. Hertzberg Y, Volovick A, Zur Y, Medan Y, Vitek S, Navon G. Ultrasound focusing using magnetic resonance acoustic radiation force imaging: application to ultrasound transcranial therapy. Med Phys. 2010;37(6):2934-42.
7. Payne A, Merrill R, Minalga E, et al. Design and characterization of a laterally mounted phased-array transducer breast-specific MRgHIFU device with integrated 11-channel receiver array. Med Phys. 2012;39(3):1552-60.
8. de Bever JT, Odeen H, Hofstetter LW, Parker DL. Simultaneous MR thermometry and acoustic radiation force imaging using interleaved acquisition. Magn Reson Med. 2017.
9. Almquist S, Parker DL, Christensen DA. Rapid full-wave phase aberration correction method for transcranial high-intensity focused ultrasound therapies. J Ther Ultrasound. 2016;4:30.
10. Vyas U, Christensen D. Ultrasound beam simulations in inhomogeneous tissue geometries using the hybrid angular spectrum method. IEEE Trans Ultrason Ferroelectr Freq Control. 2012;59(6):1093-100.
11. Svedin BT, Beck MJ, Hadley JR et al. Focal point determination in magnetic resonance-guided focused ultrasound using tracking coils. Magn Reson Med. 2016.
Figures