1192
An experimental model for mild hyperthermia with predictive temperature control in osteolytic bone metastases, using MR-guided focused ultrasound.1Radiology, Geneva University Hospitals, Image guided Interventions Laboratory, Geneva, Switzerland, 2Radiation Oncology, Geneva University Hospitals, Geneva, Switzerland
Synopsis
Combination of hyperthermia with ionizing radiation is strongly compelling, based on principles of classic radiobiology, molecular biology, and tumor physiology. MR-guided focused ultrasound (MRgFUS) is a “touch-less” approach already employed for ablative pain palliation of symptomatic bone metastases (SBM). MRgFUS mild hyperthermia adjuvant to radiation therapy has not been reported for SMB pain palliation. We optimized here the geometry of MRgFUS sonication and the automatic temperature control during steady-state long lasting hyperthermia using a realistic ex-vivo anatomic model mimicking osteolytic bone tumors. The results demonstrated uniform spatio-temporal heating, together with predictable and safe thermal condition of the cortical bone.
Background
Metastatic disease of the bone is a common cause of pain and other significant symptoms with a detrimental impact into quality of life. Single-dose palliative external beam radiation therapy is the preferred treatment option, however, complete pain response is often suboptimal and retreatment is necessary in one third of the patients. Combination of hyperthermia with radiation is strongly compelling as it is based on principles of classic radiobiology, molecular biology, and tumor physiology1-4. Ablative MR-guided focused ultrasound (MRgFUS) yielding temperature range > 65°C is already employed standalone for primary treatment of pain palliation for symptomatic bone metastases (SBM) using ExAblate5,6 (Insightec) and Sonalleve7 (Profound) devices. The response rate at 3 months is on the order of 64% with treatment-related adverse events occurring approximately in half of cases. MRgFUS mild hyperthermia adjuvant to radiation therapy has not been reported for pain palliation of SBM. A typical treatment plan requires steady-state local hyperthermia during 30 to 60 minutes. The local geometry of osteolytic bone metastases and the specific interaction of focused ultrasound with the osteal tissue may impare the intra-operatory control of isotherms.Methods
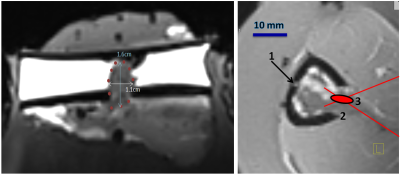
Fresh ex-vivo lamb shanks (N=8) were prepared under degassed water. After skin and muscle incision, the posterior tibial cortex was perforated using cylinder or cone drills (diameter range 4–15 mm). The yellow marrow was excavated via the cortical breakthrough. The short diameter of the cavity ranged 7.6-15.5 mm and the long diameter ranged 9.2-18.4 mm. The medullar cavity was filled with thermo-acoustic tissue-mimicking gel. The mixture is liquid above 50°C and sets as gel less than one minute after injection. The incision path was sealed with polymer suture. Targeting was achieved with high resolution T1 3D MR images of 0.8mm isotropic voxel (Figure 1) inside a 3T clinical scanner, using a receive only loop coil (11cm diameter). A fluoroptical temperature sensor (0.9mm diameter) was inserted mid-depth in the cortical bone opposite to the breakthrough site, as gold-standard safety monitor. The sonication was produced by a phased-array randomized transducer (256 element, f=1.031MHz, R=130mm, D=140mm). The site of cortical breakthrough was centered as precise as possible on the FUS beam axis. In successive experiments, the focal point was positioned 5mm ahead the cortical breakthrough site, co-planar with the site and finally 5 mm deep inside the medullar cavity. Standard PRFS thermometry using a segmented GRE-EPI sequence with lipid signal suppression and Bo-drift compensation was performed parallel and perpendicular to the bone axis (TE=10ms, voxel size 1x1x4 mm, temporal resolution 1.6s). Prescribed hyperthermia was defined as uniform +6°C temperature elevation within the tissue-mimicking gel for 12 minutes. A predictive temperature controller was implemented, automatically adjusting the FUS energy deposition. Two thermal sources were considered inside the medullar cavity: 1) the direct absorption of FUS beam around the focal point, and 2) the passive heat flow centripetally from the internal cortical wall, that is heated itself by the post-focal FUS beam. The second mechanism yields a temporal lag between the acoustic power command and the temperature response. The temperature controller took into account the predicted asymptotic level of temperature elevation, as determined from a sliding temporal window of observation.Results
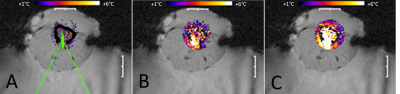
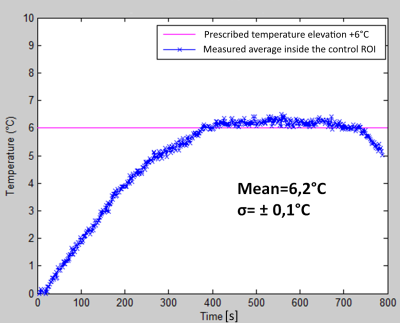
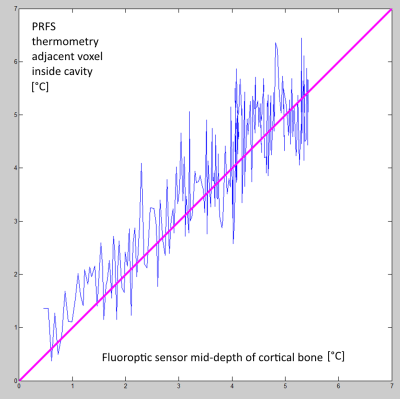
High resolution MR thermometry demonstrated that the thermal build up inside the medullar cavity tends to approximately uniform isotherms (Figure 2), as an effect of heat diffusion and circumferential heating of the surrounding cortical bone. The optimal focal point positioning was co-planar with the cortical breakthrough, without measurable thermal risk to the exposed cortical bone. Deeper positioning yielded preferential heating of the opposite cortex wall while more proximal positioning yielded enhanced heating of the breakthrough edges. The average steady-state temperature elevation in 14 procedures was 6.16+/-0.23°C (Figure 3). PRFS thermometry applied to a gel-filled voxel inside the medullar cavity, chosen to be adjacent to the intra-cortical fluoroptical sensor, was found to slightly overestimate the true temperature elevation in the post focal cortical bone (mean deviation 0.75°C, min -0.5°C, max 1.7°C). A correlation plot is provided in Figure 4.Discussion
The temporal lag between the acoustic source command and the intra-medullar temperature response produces periodic oscillations of the controlled temperature whenever a classic feedback algorithm, i.e. PID, is used, as we actually observed in preliminary experiments. This effect is theoretically explained using the Laplace-transform formalism. Our predictive controller suppressed the fluctuations and demonstrated typical accuracy of 0.2°C clearly sufficient for clinical application. While cortical bone is not directly accessible to MR thermometry, PRFS temperature monitoring of adjacent tumoral tissue was a very good substitute under the present conditions of heating.Acknowledgements
No acknowledgement found.References
1. Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiation research 2001;155:515-528.
2. Raaphorst GP, Yang DP, Ng CE. Effect of protracted mild hyperthermia on polymerase activity in a human melanoma cell line. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group 1994;10:827-834.
3. Raaphorst GP, Ng CE, Yang DP. Thermal radiosensitization and repair inhibition in human melanoma cells: A comparison of survival and DNA double strand breaks. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group 1999;15:17-27.
4. Rau B, Gaestel M, Wust P, Stahl J, Mansmann U, Schlag PM, Benndorf R. Preoperative treatment of rectal cancer with radiation, chemotherapy and hyperthermia: Analysis of treatment efficacy and heat-shock response. Radiation research 1999;151:479-488.
5. Napoli A, Anzidei M, Marincola BC, Brachetti G, Ciolina F, Cartocci G, Marsecano C, Zaccagna F, Marchetti L, Cortesi E, Catalano C. Primary pain palliation and local tumor control in bone metastases treated with magnetic resonance-guided focused ultrasound. Invest Radiol. 2013 Jun;48(6):351-8.
6. Hurwitz MD, Ghanouni P, Kanaev SV, Iozeffi D, Gianfelice D, Fennessy FM, Kuten A, Meyer JE, LeBlang SD, Roberts A, Choi J, Larner JM, Napoli A, Turkevich VG, Inbar Y, Tempany CM, Pfeffer RM. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst. 2014 Apr 23;106(5).
7. Merel
Huisman, Mie K Lam, Lambertus W Bartels, Robbert J Nijenhuis, Chrit T Moonen,
Floor M Knuttel, Helena M Verkooijen, Marco van Vulpen, Maurice A van den
Bosch. Feasibility of volumetric MRI-guided high intensity focused ultrasound
(MR-HIFU) for painful bonemetastases. J Ther
Ultrasound. 2014; 2: 16. Published online 2014 Oct
10. doi: 10.1186/2050-5736-2-16
Figures