1191
Interleaved scanning for MR-guided High Intensity Focused Ultrasound mediated drug delivery1Oncology Solutions, Philips Research, Cologne, Germany, 2Biomedical NMR, Eindhoven University of Technology, Eindhoven, Netherlands, 3Philips Research, Hamburg, Germany, 4Department of Radiology, Experimental Imaging and Image-guided Therapy, University Hospital of Cologne, Cologne, Netherlands
Synopsis
MR-guided High Intensity Focused Ultrasound (MR-HIFU) is a method that allows non-invasive heating of lesions to well-controlled ablative or hyperthermic temperatures. Control of acoustic power and focus position is achieved using a feedback based on MR thermometry. Local hyperthermia can be used as a trigger for image guided drug delivery using temperature sensitive liposomes co-encapsulating doxorubicin and MR contrast agent. The challenge is to acquire both temperature and R1-maps during hyperthermia dynamically. We investigated a novel MR method interleaving both acquisitions, with their own temporal resolution, without compromising temperature feedback showing a gradually increase of contrast agent inside rat tumors.
Purpose/introduction
MR-guided High Intensity Focused Ultrasound (MR-HIFU) mediated drug delivery has the promise to enhance the intratumoral drug concentration, increasing the therapeutic window of chemotherapeutics. This method is based on MR-HIFU induced hyperthermia as a trigger for temperature sensitive liposomes (TSLs) to release its encapsulating drug locally. Based on the release of both an MR contrast agent and a drug from TSLs, MR is able to image the release distribution using R1-mapping (R1=1/T1). In previous studies (1,2) the distribution could only be imaged post treatment. We propose a novel method where temperature mapping, necessary for controlling the hyperthermia, is interleaved with R1-mapping enabling to image the co-release during hyperthermia.Methods
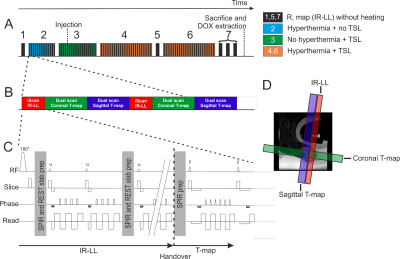
TSLs composed of DPPC:HSPC:Chol:DPPE-PEG2000 (50:25:15:3 molar ratio) were prepared encapsulating doxorubicin (dox) and ProHance® (3). Fisher 344 rats (age 11 ± 1 weeks) bearing a subcutaneous 9L gliosarcoma tumor were divided into a control (N=4, no HIFU) and treatment group (N=4). When the tumor size reached > 900 mm3, the animal was enrolled in the study. During anesthesia the tumor was depilated and the animal received analgesia and was placed in a small animal HIFU setup which was positioned on a 3T Philips Sonalleve MR-HIFU tabletop (4). Body temperature, water temperature near the tumor and respiration were continuously monitored. Figure 1A shows graphically the experimental outline of this study starting with testing if heat and Gd loaded TSL interfere with the R1 and temperature maps, respectively (2 and 3), followed by two times 15 min MR-HIFU hyperthermia (4 and 6; 8 mm treatment cell; frequency 1.4 MHz; acoustic power 14W). Non-interleaved R1 maps were acquired for comparison at baseline temperature. To enable interleaved scanning the MR patch MISS/iScan (5) was modified for external control of interleaved scans. The interleaved sequence group contained an Inversion Recovery-Look-Locker sequence (IR-LL) as “iScan” and Proton Resonance Frequency Sequence (PRFS) as externally controlled “Dual scan” (see Figure 1B and C), for R1 and temperature mapping respectively. Per dynamic cycle, one inversion recovery curve was sampled and two PRFS slices (sagittal and coronal) were acquired resulting in temporal resolution of 2 min and 8.6 s, respectively. To prevent signal saturation the IR-LL slice was placed adjacent to the sagittal temperature map (Figure 1D). After the experiment, the tumor was excised for dox quantification using high-performance liquid chromatography (doxorubicinmeasured). Whole tumor time-averaged R1,avg were calculated from R1 maps which were derived from IR-LL acquired apparent R1-maps (6). R1 values were then used to calculate the dox concentration (doxorubicincalculated) from the intraliposomal Gd/dox ratio.Results
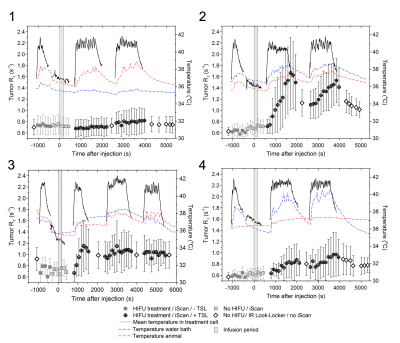
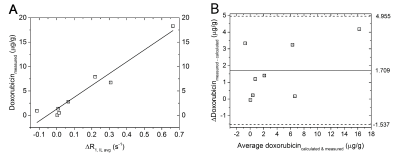
Figure 2 shows the average tumor R1 and temperature inside the treatment cell as well as the body temperature (red) and the water temperature near the tumor (blue) of all four treated animals. The interleaving of the IR-LL scans with PRFS scans did not hamper the temperature control during hyperthermia. The change in R1,avg (ΔR1, IL avg) correlated with the doxorubicinmeasured (Figure 3A, R2adj = 0.933, p = 6.1∙10-5). A Bland-Altman analysis (Figure 3B) indicated that on average, the difference between the doxorubicincalculated and the doxorubicinmeasured was 1.71 μg/g (95% CI ranging from −1.54 to 4.96 μg/g). During the experiment of animal 1, the water in which the tumor was submerged had cooled down (~ 35°C) resulting no release of Gd from the TSLs.
Conclusion
In this study, we successfully demonstrated the implementation of an interleaved scanning approach during MR-HIFU induced hyperthermia. The interleaved acquired temperature maps provided the HIFU console sufficiently feedback to ensure stable hyperthermia, while the R1 maps allowed visualizing the co-release of doxorubicin and ProHance® in the tumor. A good correlation was found between R1 change and doxorubicin concentration. The interleaved scanning approach as opposed to a single sequence allowed free choice of MRI sequences and independent optimization of scan parameters, however, possible spin history effects need to be avoided.Acknowledgements
We would like to thank NanoNextNL Project 03D.02, BMBF “TSL-LIFU” no. 3XP5014C and the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 603028 (iPaCT project) for funding. Furthermore, Jeannette Smulders and Saskia Tromp - van den Bogaerdt are acknowledged for the ICP-OES measurements (all Materials Analysis, Philips Research, Eindhoven, the Netherlands). Furthermore, we would like to thank David Veraart, Carlijn van Helvert, Leonie Niesen and Jo Habets (all Maastricht University, Maastricht, the Netherlands) and Marije Janssen (Eindhoven University of Technology) for the support with the animal experiments.References
1. Hijnen N, Kneepkens E, de Smet M, Langereis S, Heijman E, Grüll H. Thermal combination therapies for local drug delivery by magnetic resonance-guided high-intensity focused ultrasound. Proceedings of the National Academy of Sciences 2017;114:E4802. doi: 10.1073/pnas.1700790114. 2. de Smet M, Heijman E, Langereis S, Hijnen NM, Grüll H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: an in vivo proof-of-concept study. 2010;150:102–10. doi: 10.1016/j.jconrel.2010.10.036. 3. de Smet M, Langereis S, van den Bosch S, Grüll H. Temperature-sensitive liposomes for doxorubicin delivery under MRI guidance. 2009;143:120–7. doi: 10.1016/j.jconrel.2009.12.002. 4. Hijnen NM, Heijman E, Köhler MO, Ylihautala M, Ehnholm GJ, Simonetti AW, Grüll H. Tumour hyperthermia and ablation in rats using a clinical MR‐HIFU system equipped with a dedicated small animal set‐up. International Journal of Hyperthermia [Internet] 2012;28:141–155. doi: 10.3109/02656736.2011.648137. 5. Henningsson M, Mens G, Koken P, Smink J, Botnar RM. A new framework for interleaved scanning in cardiovascular MR: Application to image-based respiratory motion correction in coronary MR angiography. Magn Reson Med 2014;73:692–6. doi: 10.1002/mrm.25149. 6. Shin W, Gu H, Yang Y. Fast high-resolutionT1mapping using inversion-recovery look-locker echo-planar imaging at steady state: Optimization for accuracy and reliability. Magn Reson Med [Internet] 2009;61:899–906. doi: 10.1002/mrm.21836.Figures