1189
In vivo MR-ARFI for transcranial focused ultrasound in large animalsPooja Gaur1, Ningrui Li2, Rachelle Bitton1, and Kim Butts Pauly1
1Radiology, Stanford University, Stanford, CA, United States, 2Electrical Engineering, Stanford University, Stanford, CA, United States
Synopsis
Focused ultrasound through the skull is desirable for noninvasive brain therapies such as neuromodulation. However, the skull distorts the ultrasound beam and absorbs varying amounts of energy depending on bone thickness and composition. In light of these challenges, we investigate magnetic resonance acoustic radiation force imaging (MR-ARFI) focal spot imaging of subcortical brain tissue in a large animal, and simulations and measurements of acoustic pressure transmitted through the skull. Results show in vivo MR-ARFI focal spot imaging through the skull for the first time, and provide practical information on focal spot targeting through skulls of varying shape, thickness, and composition.
Introduction
Magnetic resonance imaging (MRI)-guided transcranial focused ultrasound noninvasively delivers targeted ultrasound energy through the skull to specific locations in the brain. However, the skull poses a major challenge by distorting and attenuating the ultrasound beam in patients, where pressure can vary by 4x1,2, and even in rodent models where the skull is thin3. It is therefore critical to plan ultrasound targeting in the brain and to verify the targeting with imaging. In nonthermal applications such as neuromodulation4-6, targeting without tissue heating can be done with magnetic resonance acoustic radiation force imaging (MR-ARFI)7. In a large animal model, we demonstrate in vivo focal spot targeting using MR-ARFI, ex vivo focal spot targeting across skull caps, and simulations and measurements of acoustic pressure transmitted through the skull. Results provide practical information on focal spot targeting through skulls of varying shape, thickness, and composition.Methods
All experiments used a 1024 element, 550 kHz focused ultrasound transducer (ExAblate 2100, Insightec Ltd., Haifa, Israel). MRI-guided focused ultrasound was performed at 200 W (in vivo) and 170 W (ex vivo) acoustic power.MR-ARFI FOCAL SPOT IMAGING
A 30 kg anesthetized male Dorset cross sheep was imaged at 3T (Signa Excite, GE Healthcare, Milwaukee, WI) with the transducer positioned above the head for targeting subcortical brain tissue prior to neuromodulation. MR-ARFI data were acquired using a 2DFT spin echo sequence with repeated bipolar motion encoding gradients and 500 ms repetition time (TR), 39 ms echo time, 20 x 20 x 0.7 cm3 field of view, and 256 x 128 acquisition matrix8. Ultrasound spanned 16 ms per TR across the second lobe of the first bipolar, the refocusing pulse, and the first lobe of the second bipolar gradient to encode ultrasound-induced tissue displacement as a phase shift. Two MR-ARFI data acquisitions with reversed motion encoding gradient polarities were combined to form the displacement phase image. Following the acute study, the skull cap was extracted.
Three ex vivo sheep skull caps, roughly 3.5 mm, 5.35 mm, and 5.4 mm in thickness, were positioned on a 4 inch diameter gel phantom and submerged in a water-filled cylinder, with the transducer positioned above. Focused ultrasound was targeted to locations over a 5x5 grid ranging from +/-20 mm in x and y, and imaged with the same MR-ARFI parameters as the in vivo study. The same measurements were collected in the phantom alone, and the ratios of peak displacement with and without the skull caps were computed at each location.
SIMULATING AND MEASURING ACOUSTIC PRESSURE
Computed tomography (CT) images of each skull cap were acquired at 120 kVp with a bone filter. Focal spot pressure was simulated using CT-derived estimates of skull acoustic properties and the hybrid angular spectrum method2,9.
Four ex vivo sheep skull caps (the three above, and a fourth with 7 mm thickness) were degassed, affixed to the transducer, and placed in a water tank. A fiber optic hydrophone (Precision Acoustics, Dorchester, UK) directly measured acoustic pressure at the focal spot for sonications between 0 and 200 W acoustic power.
Results and Discussion
MR-ARFI results show in vivo focal spot imaging of subcortical brain tissue through the skull in an alive large animal, to our knowledge for the first time (Fig. 1).Peak MR-ARFI displacement through each ex vivo skull cap was greatest where the skull was comparatively flat and thin (Fig. 2). Although the ex vivo skull caps in Fig. 2(b)-(c) are nearly equivalent in thickness, that in Fig. 2(b) had higher relative displacement and higher transmitted pressure (Fig. 3).
Skull thickness measures are a starting point for estimating acoustic pressure at a given power level (Figs. 3-4). MR-ARFI provides additional information relating to focal spot intensity, including how it varies through each skull based on its shape and its thickness. Simulations incorporate acoustic properties of the skull, providing important pre-treatment information. Variation in bone composition, indicated by distribution of Hounsfield units across the skull caps (Fig. 5), provides additional insight on MR-ARFI and hydrophone focal spot measurements.
Results highlight the need for treatment planning that is specific to individual skull properties, such as beam simulation10,11, as thickness alone is insufficient for predicting focused ultrasound transmission through the skull. Future work will include more samples, additional simulation studies, and reproducibility studies. Our findings show MR-ARFI is valuable for imaging focal spot location and pressure through the skull, and enables non-invasive, non-thermal focal target verification in vivo.
Acknowledgements
This work was supported by NIH T32 EB009653, T32 CA009695, R01 MH111825, R01 EB019005, and P01 CA 159992. The authors would like to thank Yamil Saenz for help with sheep studies, Elias Godoy for help with tissue recovery, Jan Kubanek for help with experiments, and Lior Molvin for help with CT scans.References
[1] Pauly KB. Metric for comparison of treatment efficiency in transcranial MR-guided focused ultrasound. In 15th International Symposium on Therapeutic Ultrasound, Utrecht. 2015; p. 73.[2] Vyas U, et al. Predicting variation in subject thermal response during transcranial magnetic resonance guided focused ultrasound surgery: Comparison in seventeen subject datasets. Med Phys 2016;43:5170–5180.
[3] Gerstenmayer M, et al. Magnetic resonance acoustic force imaging for in vivo estimation of ultrasonic transmission factor through rat skulls. In 16th International Symposium on Therapeutic Ultrasound, Tel Aviv. 2016; p. 95.
[4] Lee W, et al. Image-guided focused ultrasound-mediated regional brain stimulation in sheep. Ultrasound Med Biol 2016;42:459-470.
[5] Dallapiazza RF, et al. Noninvasive neuromodulation and thalamic mapping with low-intensity focused ultrasound. J Neurosurg 2017:1-0.
[6] Caskey CF, et al. Ultrasound Neuromodulation with MRI for Brain Circuitry in Non-Human Primates. In Proc Intl Soc Mag Reson Med 25, Honolulu. 2017.
[7] McDannold N, Maier SE. Magnetic resonance acoustic radiation force imaging. Med Phys 2008;35:3748-3758.
[8] Bitton RR, et al. Toward MR-guided high intensity focused ultrasound for presurgical localization: Focused ultrasound lesions in cadaveric breast tissue. JMRI 2012;35:1089–1097.
[9] Vyas U, Christensen D. Ultrasound beam simulations in inhomogeneous tissue geometries using the hybrid angular spectrum method. IEEE Trans Ultrason Ferroelectr Freq Control 2012;59:1093–1100.
[10] Odéen H, et al. MR thermometry for focused ultrasound monitoring utilizing model predictive filtering and ultrasound beam modeling. J Ther Ultrasound 2016;4:23.
[11] Top CB, et al. Nonthermal ablation of deep brain targets: a simulation study on a large animal model. Med Phys 2016;43:870-882.
Figures
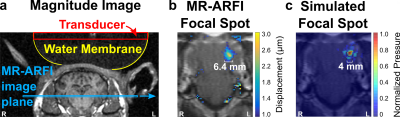
Figure 1. In vivo focal spot targeting with MR-ARFI. (a) Magnitude image showing ultrasound transducer coupled to the sheep by a water membrane, and slice location for focal spot imaging. (b) Overlay of MR acoustic radiation force (MR-ARFI) map on magnitude image, showing the ultrasound focal spot (masked in brain; cropped to show detail). (c) Focal spot simulation over MR-ARFI magnitude image. The full width at half maximum is reported below MR-ARFI and simulated focal spots (b-c).
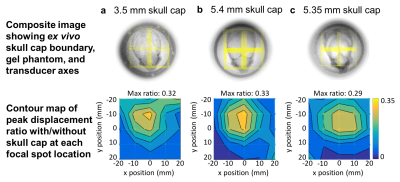
Figure 2. Focal spot steering across ex vivo skull caps. Top row: top-down view of phantom setup for three ex vivo skull caps (a-c) showing skull cap position over gel-filled cylinder and overlay of transducer x,y axes (yellow). Bottom row: Ratio of peak displacement at each focal spot location relative to phantom-only measurements. Peak displacement is highest along the thinner, flatter region for each ex vivo skull cap.
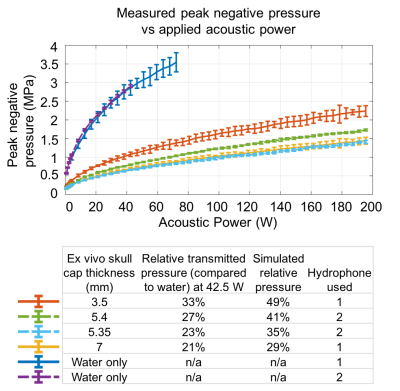
Figure 3. Measured and simulated acoustic pressure. Top: Peak negative pressure measured by hydrophones with and without skull caps. Bottom: Transmitted pressure through each skull cap relative to measurements in water and simulated relative pressure. Simulated pressure estimates were higher than measured values. However, both measured and simulated pressure decreased on the order of 20% between 3.5 mm and 5.4 mm thick skull caps, and between 5.4 mm and 5.35 mm thick skull caps.
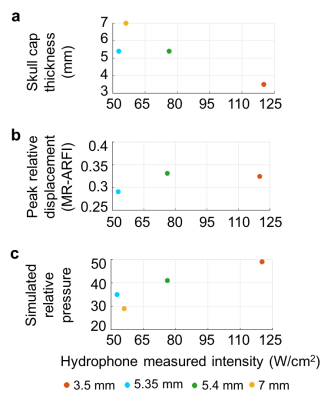
Figure 4. (a) Skull cap thickness, (b) peak MR-ARFI displacement, and (c) simulated relative pressure vs hydrophone measured intensity at 170 acoustic W for each skull cap.
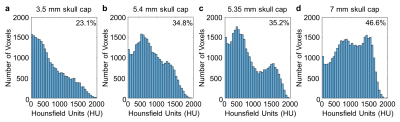
Figure 5. Distribution of Hounsfield units (HU) from CT images in a region covering the top of each skull cap. Percentage of voxels corresponding to skull based on HU > 500 (top right of histograms) is similar to measured thickness. Although skull caps in (b) and (c) are nominally similar, histograms show variations in Hounsfield unit distributions. The sample in (c) has more apparent variation in bone composition, with higher trabecular bone (500-1000 HU) compared to (b), which may contribute to the differences in their transmitted ultrasound measurements.