1187
Feasibility of Blood-Brain Barrier Opening in Patients with Alzheimer’s Disease by MR-Guided Focused Ultrasound1Sunnybrook Research Institute, Toronto, ON, Canada, 2Division of Neurosurgery, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 3Hurvitz Brain Sciences Research Program, Sunnybrook Research Institute, Toronto, ON, Canada, 4Division of Neurology, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 5Division of Geriatric Psychiatry, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 6Department of Medical Imaging, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 7Department of Psychiatry and Behavioral Sciences and Radiology and Radiological Sciences, Johns Hopkins University, Baltimore, MD, United States, 8Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
In a phase I clinical trial, the feasibility and safety of focal blood-brain barrier (BBB) opening in patients with Alzheimer’s disease using the ExAblate focused ultrasound system was evaluated. Six patients were treated in the white matter of the prefrontal cortex. It was demonstrated that BBB opening can be achieved without any red-blood-cell extravasation.
Introduction
For treating Alzheimer’s disease, studies in transgenic mouse models have shown that blood-brain barrier (BBB) opening by focused ultrasound (FUS) results in significant reductions in amyloid as well as a reversal of memory deficits1,2. In this phase I trial, the feasibility and safety of focal BBB opening in patients with Alzheimer’s disease using the ExAblate FUS system was evaluated.Methods
Six patients with mild-to-moderate Alzheimer’s disease were investigated. The study was approved by the institutional Research Ethics Board. A modified clinical MR-guided FUS brain system (ExAblate 4000, 230 kHz, InSightec, Tirat Carmel, Israel) was used with a 3T MR scanner (Signa MR750, GE Healthcare, Milwaukee, WI, USA). The patient's head was shaved and positioned in the FUS array with a stereotactic frame. To minimize potential complications in the event of bleeding, a non-eloquent brain region of 5 or 9 mm in diameter in the white matter of the dorsolateral prefrontal cortex was selected as the target, avoiding sulci and large vessels. The focus was steered during sonications over a 2x2 grid with 2.5mm spacing or a 3x3 grid with 3mm spacing, depending on the size of the target volume. The overall pulse repetition frequency of FUS was 0.9%. A bolus injection of 4 ul/kg of Definity microbubbles (Lantheus Medical Imaging, N. Billerica, MA, USA) was applied simultaneously with each sonication (1/5th of the clinical dose for ultrasound imaging). With the first injection of microbubbles, a ramp sonication with 5% step-size in incremental power was applied to find the appropriate power level based on feedback of cavitation signals. Cavitation signals were detected by six acoustic receivers and a cavitation threshold was established based on a pre-clinical study3. Once clear cavitation was detected at a certain power level, a 100 s sonication over the grid at half of the power level4 was applied with a separate injection of microbubbles for BBB opening. Post sonication, Gd (Gadovist, Bayer)-enhanced 3D FSPGR images were acquired to verify the BBB openings, and T2*-weighted GRE images (TE=15ms) were collected to detect potential hemorrhage. After the treatment, the patient was released from the head frame and MR scans were repeated with an 8-channel head coil for better quality images. A second treatment was performed one month later at the same target plus an adjacent volume to evaluate the feasibility of repeated and larger BBB opening volumes.Results
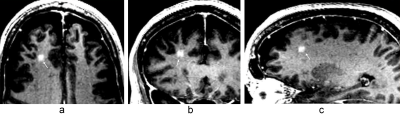
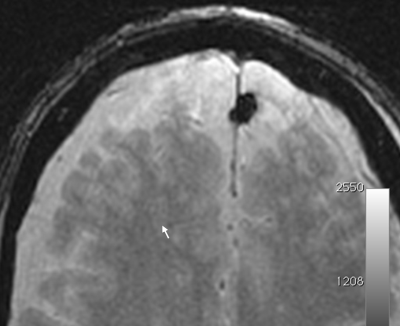
BBB opening was successfully achieved demonstrated by Gd enhancement in the treatment area with a signal intensity increase of 35 ± 22%. Hypointensities detected on T2*w images were observed for the first subject indicating microscopic hemorrhage. Subsequently, the cavitation threshold was lowered and no further hypointensity was observed on following treatments. Figure 1 shows Gd enhancement after BBB opening of a 2x2 grid in 3-plane views. Figure 2 shows no hypointensity signal in the T2*w image at the BBB opened volume. At the second treatment, BBB was successfully opened again in 5 patients (1 pending).Discussion
In an earlier reported case of BBB opening in brain tumors5, BBB opening was accompanied by minor red-blood-cell (RBC) extravasations. Since then a ramp sonication was developed with power increments in finer steps so that the proper power level could be detected by cavitation signals with better accuracy. With the current study in Alzheimer's patients, we demonstrated that BBB opening can be achieved without any RBC extravasation.
For this study, we targeted a non-eloquent brain region. With the establishment of the feasibility and safety profile, further investigation is warranted to target potentially more eloquent areas such as the hippocampus6 and other cortical and subcortical structures.
Acknowledgements
The authors thank the Focused Ultrasound Foundation for funding this trial and InSightec for technical supports of the ExAblate system. The development of this method was funded by NIH grant no. EB003268.References
1. Jordao JF, Thevenot E, Markham-Coultes K, et al. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol 2013;248:16-29.
2. Leinenga G and Gotz J. Scanning ultrasound removes amyloid-b and restores memory in an Alzheimer’s disease mouse model. Sci Transl Med 2015;7:278ra33.
3. Huang Y, Alkins R, Schwartz ML, et al. Opening the blood-brain barrier with MR imaging-guided focused ultrasound: preclinical testing on a trans-human skull porcine model. Radiology 2017;282:123-130.
4. O’Reilly MA and Hynynen K. Blood-brain barrier: real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions–based controller. Radiology 2012;263:96-106.
5. Huang Y, Alkins R, Chapman M, et al. Initial experience in a pilot study of blood-brain barrier opening for chemo-drug delivery to brain tumors by MR-guided focused ultrasound. ISMRM 2016, abstract 450.
6. Burgess A, Dubey S, Yeung S, et al. Alzheimer disease in a mouse model: MR imaging–guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology 2014; 273:736–45.