1185
Free-breathing R2* Mapping in the Entire Placenta During Early Gestation Using 3D Stack-of-Radial MRI at 3 T: Investigation of Spatial and Temporal Variation1Radiological Sciences, University of California Los Angeles, Los Angeles, CA, United States, 2Physics and Biology in Medicine, University of California Los Angeles, Los Angeles, CA, United States, 3Epidemiology, University of California Los Angeles, Los Angeles, CA, United States, 4Pediatrics, David Geffen School of Medicine at UCLA, University of California Los Angeles, Los Angeles, CA, United States, 5Obstetrics and Gynecology, David Geffen School of Medicine at UCLA, University of California Los Angeles, Los Angeles, CA, United States
Synopsis
Current methods for detecting ischemic placental disease are either invasive or have low sensitivity. MRI can be used to non-invasively characterize tissue hypoxia with R2* mapping. However, conventional Cartesian MRI methods are sensitive to motion artifacts due to maternal and fetal motion. In this study, a non-Cartesian free-breathing 3D stack-of-radial MRI technique (FB radial) for R2* mapping in the placenta during early gestation was investigated at 3T. In 20 subjects, placental R2* range, accuracy, repeatability, spatial variation, and temporal variation were analyzed. Results demonstrate that FB radial is an accurate and repeatable technique for R2* mapping in the entire placenta.
Introduction
Ischemic placental disease (IPD) can lead to pre-eclampsia, fetal growth restriction, preterm labor, and spontaneous abortion(1,2). Detection of IPD during early gestation may enable earlier intervention to improve outcomes. Current methods to detect IPD include invasive chorionic villous sampling (CVS)(3) and non-invasive uterine artery Doppler (UA Doppler). However, CVS can cause complications and UA Doppler has low sensitivity during early gestation(4,5). MRI may be able to detect IPD by characterizing hypoxia with R2* maps(6–8). However, conventional R2* mapping techniques employ Cartesian sampling, which is sensitive to coherent aliasing artifacts caused by maternal respiratory motion, uterine contractions, and fetal motion. Therefore, previous studies have used 2D breath-held Cartesian techniques, which limits the placental coverage, potentially causing sampling bias and reducing repeatability(6–8). 3D stack-of-radial trajectories have greater robustness to motion artifacts, allowing for free-breathing MRI(9). In this work, we propose and evaluate a free-breathing (FB) 3D stack-of-radial technique(10,11) for quantifying R2* in the entire placenta at 3T during early gestation.Methods
FB Radial Sequence A bipolar multiecho gradient echo 3D stack-of-radial sequence with golden-angle ordering (FB radial) (Fig. 1a)(10,11) was developed to improve robustness to motion and enable R2* mapping of the entire placenta.
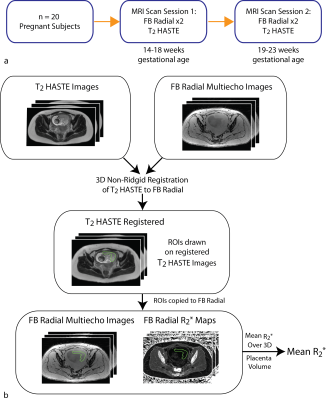
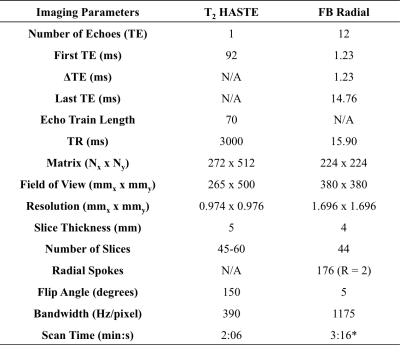
Experimental Design A phantom with an R2* range of 5-70s-1 was scanned with FB radial and a multiecho gradient echo Cartesian sequence (Cartesian) with matched imaging parameters (Table 1). With IRB approval and after obtaining informed consent, twenty pregnant subjects (age=35.41±3.41years) without any known pregnancy complications were enrolled. Placenta scans were acquired with subjects in the feet-first supine position at 3T (Skyra/Prisma, Siemens) using 2D T2 HASTE(12) and FB radial sequences (Table 1). Each subject had scans performed at two gestational age (GA) intervals of 14-18 weeks and 19-23 weeks (Fig. 1a). Two FB radial scans were acquired back-to-back in the same session to assess repeatability.
Reconstruction FB radial and Cartesian images were reconstructed offline. FB radial reconstruction included gradient calibration and correction(11). Signal model fitting for both FB radial and Cartesian was performed(13–15), a 7-peak fat model(16), and a single effective R2* per voxel(17–19).
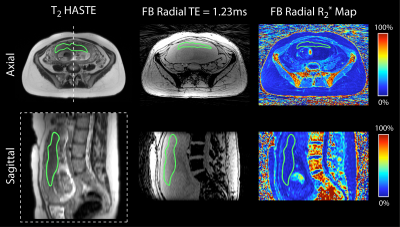
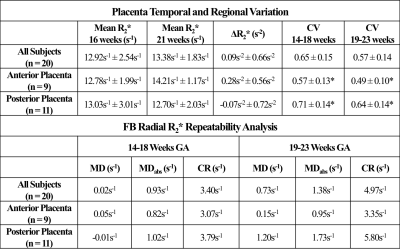
Analysis T2 HASTE images were registered to FB radial images using Advanced Normalization Tools software(20). The entire placenta was contoured on registered T2 HASTE images and regions of interest (ROIs) were copied to FB radial images and R2* maps (Fig. 1b). FB radial R2* repeatability was assessed by calculating the mean difference (MD), absolute mean difference (MDabs), and coefficient of repeatability (CR). R2* from the FB radial scans performed at the two GA intervals were used to determine normalized mean R2* at 16 and 21 weeks GA, mean coefficient of variation (CV) to assess spatial variation, and mean change in R2* over GA (ΔR2*) to assess temporal variation. Subjects were analyzed together and also by placenta implantation position (anterior or posterior). A two-sided t-test was performed to compare differences in mean R2*, CV, and ΔR2* between anterior and posterior placenta positions (P<0.05 considered significant).
Results
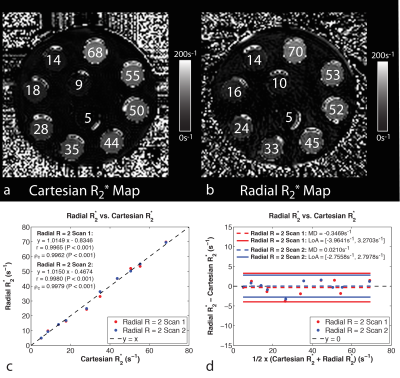
FB radial provided accurate and repeatable R2* quantification in a phantom (Fig. 2) compared to a reference Cartesian method. In pregnant subjects, images were acquired without substantial motion or undersampling artifacts using FB radial (Fig. 3). FB radial R2* had a range of 9-21s-1 and demonstrated repeatability with MD<1.2s-1, MDabs<1.75s-1 and CR<6s-1. The normalized mean R2* at 16 and 21 weeks, CV, and ΔR2* are shown in Table 2. Higher CR and CV (P<0.05) were observed for the posterior placentas. Mean R2* for posterior placentas tended to decrease as a function of GA, while mean R2* tended to increase for anterior placentas.Discussion
FB radial R2* mapping demonstrated accuracy and repeatability in a phantom, and good repeatability in pregnant subjects. FB radial improves patient comfort during MRI by not requiring breath-holding and accommodating maternal and fetal motion. By acquiring 3D R2* maps throughout the entire placenta, FB radial may reduce sampling bias and characterize spatial variation. FB radial R2* had better repeatability in the first session compared to the second one, particularly for posterior placentas. Variations may be due to fetal motion, which was observed in some repeated scans. Ongoing work will include more subjects to achieve necessary power for significance and further investigation of spatial and temporal variation. This study contributes to the understanding of R2* in normal pregnancies during early gestation and may be extended to study IPD and abnormal pregnancies for early detection and intervention. Previous studies at 1.5T during later GA showed significant R2* changes in abnormal pregnancies(6,7).Conclusion
FB radial can provide accurate and repeatable R2* mapping in the entire placenta of pregnant subjects at 3T. This technique allows for increased comfort and may support early detection and monitoring of ischemic placental disease.Acknowledgements
This work acknowledges the use of the ISMRM Fat-Water Toolbox (http://ismrm.org/workshops/FatWater12/data.htm). This work was funded by the grant NICHD U01-HD087221. The authors thank Sitaram S. Vangala at UCLA for helpful discussions.References
1. Kingdom JCP, Kaufmann P. Oxygen and placental villous development: Origins of fetal hypoxia. Placenta 1997;18:613–621. doi: 10.1016/S0143-4004(97)90000-X.
2. Parks WT. Placental hypoxia: The lesions of maternal malperfusion. Semin. Perinatol. 2015;39:9–19. doi: 10.1053/j.semperi.2014.10.003.
3. Rhoads GG, Jackson LG, Schlesselman SE, et al. The safety and efficacy of chorionic villus sampling for early prenatal diagnosis of cytogenetic abnormalities. N Engl J Med 1989;320:609–617. doi: 10.1056/NEJM198903093201001.
4. Bamfo JE a K, Odibo AO. Diagnosis and management of fetal growth restriction. J. Pregnancy 2011;2011:640715. doi: 10.1155/2011/640715.
5. Khong SL, Kane SC, Brennecke SP, Da Silva Costa F. First-trimester uterine artery doppler analysis in the prediction of later pregnancy complications. Dis. Markers 2015;2015. doi: 10.1155/2015/679730.
6. Sinding M, Peters DA, Frøkjær JB, Christiansen OB, Petersen A, Uldbjerg N, Sørensen A. Prediction of low birth weight: Comparison of placental T2* estimated by MRI and uterine artery pulsatility index. Placenta 2017;49:48–54. doi: 10.1016/j.placenta.2016.11.009.
7. Sinding M, Peters D a., Frøkjaer JB, Christiansen OB, Petersen A, Uldbjerg N, Sørensen A. Placental T2* measurements in normal pregnancies and in pregnancies complicated by fetal growth restriction. Ultrasound Obstet. Gynecol. 2015. doi: 10.1002/uog.14917.
8. Huen I, Morris DM, Wright C, Parker GJM, Sibley CP, Johnstone ED, Naish JH. R1 and R2 * changes in the human placenta in response to maternal oxygen challenge. Magn. Reson. Med. 2013;70:1427–33. doi: 10.1002/mrm.24581.
9. Block KT, Chandarana H, Milla S, et al. Towards routine clinical use of radial stack-of-stars 3D gradient-echo sequences for reducing motion sensitivity. J. Korean Soc. Magn. Reson. Med. 2014;18:87–106. doi: 10.13104/jksmrm.2014.18.2.87.
10. Armstrong T, Liu D, Martin T, et al. Free-breathing R2* Characterization of the Placenta During Normal Early Gestation Using a Multiecho 3D Stack-of-Radial Technique. In: In Proc. Intl. Soc. Mag. Reson. Med. ; 2017. p. 117.
11. Armstrong T, Dregely I, Stemmer A, Han F, Natsuaki Y, Sung K, Wu HH. Free-breathing liver fat quantification using a multiecho 3D stack-of-radial technique. Magn. Reson. Med. 2017. doi: 10.1002/mrm.26693.
12. Patel MR, Klufas RA, Alberico RA, Edelman RR. Half-fourier acquisition single-shot turbo spin-echo (HASTE) MR: Comparison with fast spin-echo MR in diseases of the brain. Am. J. Neuroradiol. 1997;18:1635–1640.
13. Hernando D, Kellman P, Haldar JP, Liang Z-P. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn. Reson. Med. 2010;63:79–90. doi: 10.1002/mrm.22177.
14. ISMRM Fat Water Toolbox. 2012.
15. Gleich DF. Models and Algorithms for PageRank Sensitivity. Stanford University; 2009.
16. Ren J, Dimitrov I, Sherry AD, Malloy CR. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. J. Lipid Res. 2008;49:2055–2062. doi: 10.1194/jlr.D800010-JLR200.
17. Horng DE, Hernando D, Hines CDG, Reeder SB. Comparison of R2* correction methods for accurate fat quantification in fatty liver. J. Magn. Reson. Imaging 2013;37:414–22. doi: 10.1002/jmri.23835.
18. Horng DE, Hernando D, Reeder SB. Quantification of liver fat in the presence of iron overload. J. Magn. Reson. Imaging [Internet] 2017. doi: 10.1002/jmri.25382.
19. Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn. Reson. Med. 2008;60:1122–34. doi: 10.1002/mrm.21737.
20. Advanced Normalization Tools ( ANTs ).
Figures