1184
4D flow MRI in renal transplant: preliminary results.1Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai Hospital, New York, NY, United States, 2Radiology, Icahn School of Medicine at Mount Sinai Hospital, New York, NY, United States, 3University Hospital Zurich, Zurich, Switzerland, 4Groupe Hospitalier Pitie-Salpetriere, Paris, France, 5Feinberg School of Medicine, Radiology, Northwestern University, Chicago, IL, United States, 6McCormick School of Engineering, Biomedical Engineering, Northwestern University, Chicago, IL, United States, 7Recanati-Miller Transplantation Institute, Icahn School of Medicine at Mount Sinai Hospital, New York, NY, United States, 8Pathology, Icahn School of Medicine at Mount Sinai Hospital, New York, NY, United States
Synopsis
In this preliminary study, we sought to determine the test-retest repeatability of flow quantification in renal allograft vessels using a 4D flow phase-contrast (PC) MRI sequence, and to correlate flow parameters with renal function. We observed significantly decreased renal arterial flow in allografts with chronic dysfunction, as well as positive correlation between flow and velocities in renal transplant vessels and renal function. We conclude that 4D flow imaging is sensitive to the vascular changes that accompany renal transplant dysfunction, to be confirmed in a larger study.
Introduction
Phase-contrast MRI is a promising method for flow quantification of renal transplant vessels, as it does not employ gadolinium-based contrast agents, but has been poorly reported. The goals of our study are to report our preliminary experience with 4D flow in renal transplants, determine the test-retest repeatability of flow quantification in renal allograft vessels using a 4D flow phase-contrast (PC) MRI sequence, and to correlate flow parameters with eGFR.Methods
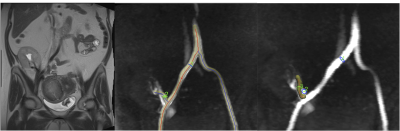
21 initial patients including 12 with functional renal allografts (M/F 6/6 mean age 54y, estimated MDRD serum eGFR 66±11 ml/min/1.73m2, range 50-87 ml/min/1.73m2), 9 with chronic dysfunction (M/F, 4/5, mean age 53.6 y, eGFR 27±15 ml/min/1.73m2, range 12-63 ml/min/1.73 m2) were enrolled in this IRB-approved single center prospective study. All patients had Cartesian 4D flow imaging at 1.5T (Aera, Siemens), as part of a multiparametric MRI protocol, which included dynamic contrast-enhanced MRI (DCE-MRI) in patients without renal dysfunction. Three patients (2 with functional allografts, 1 with chronic dysfunction without biopsy-proven fibrosis) underwent re-test imaging on a second visit 18±9 days after the first. The 4D flow acquisition consisted of a coronal-oblique abdominal 60 mm slab (TR/TE/FA 62.4/2.9/9º, FOV 400x400 mm, acquired matrix size 160 x 160 x12, acquired voxel size 2.5 x 2.5 x 5 mm3, interpolated voxel size 1.3 x 1.3 x 2.5 mm3, temporal resolution 66-71 ms), covering the renal allograft in the pelvis. 4D flow was acquired for 3 minutes during free breathing, with a velocity encoding parameter (VENC) of 120 cm/sec. Images were analyzed using prototype software (Siemens Healthcare) by 2 observers in consensus. After performing corrections for phase aliasing, eddy currents and motion, vessels surrounding the allograft, including the main stems of the renal artery (RA) and renal vein (RV) (Fig. 1), as well as the right (RILA) and left common iliac artery (LILA) were identified and segmented. Time-averaged vessel cross-section area, through-plane velocity and flow, as well as maximum velocity, were measured. Agreement on vessel identification between the test-retest sessions was evaluated by Cohen’s kappa. Test-retest agreement of area, average and peak velocity and flow measurements was evaluated by coefficient of variation (CV) and Bland-Altman statistics. Flow parameters for the segmented vessels, as well as for the iliac artery ipsilateral to the allograft (right/left iliac fossa allograft placement: 17/4 allografts) were compared between patients with functional and dysfunctional allografts by Mann-Whitney tests. Flow parameters were correlated to serum eGFR by Spearman correlation.Results
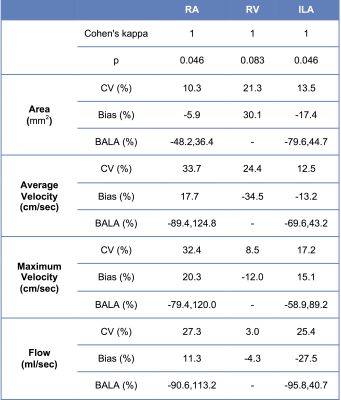
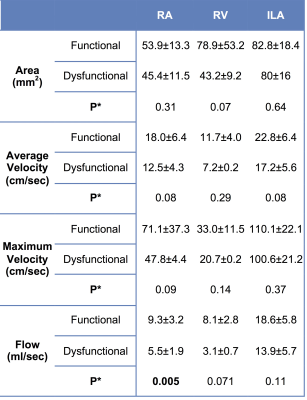
Table 1 shows test-retest statistics for individual vessels. There was excellent agreement between test-retest sessions in segmenting the vessels (Cohen’s kappa=1, p=0.046). Area, velocity and flow measurements showed acceptable to low test-retest agreement, with CV and bias <35%, but high limits of agreement. There was significantly decreased RA flow (p=0.005) in patients with allograft dysfunction (Table 2). There were trends towards significant decrease of RV area and flow, and trend towards decreased ipsilateral iliac artery (ILA) velocity and flow (Table 2). There were significant moderate positive correlations between each of RA flow (Spearman’s ρ=0.544, p=0.018) and ILA flow (Spearman’s ρ=0.457, p=0.0039) with eGFR. We observed AUC of 0.875 (p=0.006), sensitivity of 87.5% and specificity of 90.9% (using cut-off value for RA flow of 6.5 ml/s) for diagnosing allograft dysfunction.Discussion
The maximum velocities measured in the RA in our study are in agreement with a recent study performed in renal allograft recipients1. Test-retest agreement was modest in this initial study with 3 patients undergoing re-test visits almost 20 days apart. The modest agreement may be due to physiologic variation in hemodynamic state. We did not observe significant differences in the RV in this preliminary study, since our acquisition parameters (VENC= 120 cm/sec) were more sensitive to arterial flow. Data acquired with acquisition parameters sensitive to venous flow (VENC= 45 cm/sec) is forthcoming.
Factors that restrict RA flow, such as renal artery stenosis, may affect renal function and blood pressure regulation after transplantation2. The development of fibrosis with decreased renal artery flow has been shown in animal models of renal artery stenosis3. Our study shows that 4D flow can potentially be used as a non-contrast method to diagnose renal dysfunction.
Conclusion
4D flow imaging is sensitive to the vascular changes
that accompany chronic renal dysfunction, with the causal relationship between
vascular flow and renal dysfunction to be investigated in a larger mechanistic
study.Acknowledgements
This research was supported by the National Institutes of Health NIDDK Grant 1F32DK109591, Society of Abdominal Radiology (SAR) Morton Bosniak Research Award, and Guerbet LLC Grant.References
1. Motoyama D, Ishii Y, Takehara Y, et al. Four-dimensional phase-contrast vastly undersampled isotropic projection reconstruction (4D PC-VIPR) MR evaluation of the renal arteries in transplant recipients: Preliminary results. Journal of magnetic resonance imaging : JMRI 2017;46(2):595-603.
2. Sankari BR, Geisinger M, Zelch M, Brouhard B, Cunningham R, Novick AC. Post-transplant renal artery stenosis: impact of therapy on long-term kidney function and blood pressure control. The Journal of urology 1996;155(6):1860-1864.
3. Korsmo MJ, Ebrahimi B, Eirin A, et al. Magnetic resonance elastography noninvasively detects in vivo renal medullary fibrosis secondary to swine renal artery stenosis. Investigative radiology 2013;48(2):61-68.
Figures