1179
Ex vivo MRI evaluation of vulvar cancer in fresh wide local excision specimens for cancer localization and prediction of the surgical tumour free margins.1Radiology and Nuclear Medicine, Radboud university medical center, Nijmegen, Netherlands, 2Obstetrics and Gynaecology, Radboud university medical center, Nijmegen, Netherlands, 3Pathology, Radboud university medical center, Nijmegen, Netherlands, 4Operating Rooms, Radboud university medical center, Nijmegen, Netherlands
Synopsis
Currently, perioperative information on the surgical margin status of wide local excision specimens containing vulvar cancer is only based on the surgeon’s estimation. In this study we performed both a non-blinded and a blinded annotation of vulvar cancer location and surgical tumour free margins in the ex vivo MRI obtained from fresh wide local excision specimens. Annotated whole-mount section histopathology slides obtained from totally included specimens were adhered as gold standard. There was high correlation and agreement between ex vivo MRI, and high NPV and PPV were obtained for vulvar cancer localization and identification of margins <8 mm.
Introduction
Wide local excision (WLE) combined with a sentinel node procedure or inguinal lymph node dissection is the preferred treatment of the primary tumour in vulvar squamous-cell carcinoma (VSCC)1,2. Tumour free margins of ≥8 mm are considered the most important predictive factor for local recurrences3–5. The main challenge in treating VSCC is to keep the surgical margins large enough to minimize the chance of local recurrence and simultaneously preserve as much genital tissue as possible to diminish morbidity to urethra, clitoral or anal sphincter. The surgeon is hampered by the fact that no accurate and topical information on the margin status is available during or directly after the surgery. Previously optimal imaging was investigated6, whereas this study assessed the value of ex vivo MRI in providing information on VSCC location and the surgical tumour free margin status in fresh WLE specimens. Both technical feasibility and clinical applicability were investigated.Methods
After approval
of our Institutional Review Board, written informed consent from all patients
(n=9) was obtained. Consecutive patients with biopsy proven VSCC who were
scheduled for WLE were prospectively included. Following surgery, the entire intact
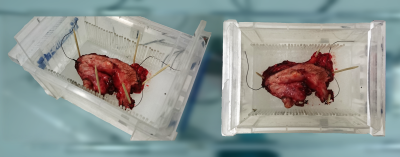
vulva specimen was positioned in a specifically designed container and was
scanned using a 7 Tesla preclinical MR scanner interfaced to a Siemens console.
The scan protocol was described previously6. After imaging, the specimens were completely cut into
3 mm thick slices and whole-mount H&E-stained slides were obtained for
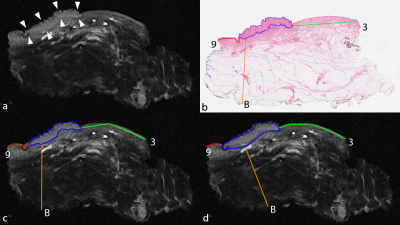
histological examination. A pathologist annotated VSCC area and minimal
histological tumour free margins (3- and 9 o’clock, and basal) on each
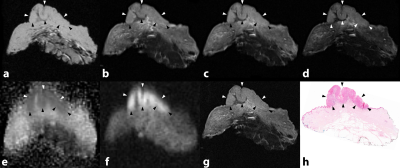
digitalized histological slide. Next, individual ex vivo MRI slices were
correlated with the histological slides. An observer with the histological
images at his disposal annotated VSCC area and minimal tumour free margins on
the correlated ex vivo MRI (the non-blinded annotation). Subsequently, a
radiologist performed annotation of the same features as well, however, without
knowledge of the histology (the blinded annotation). The ex vivo MRI measurements
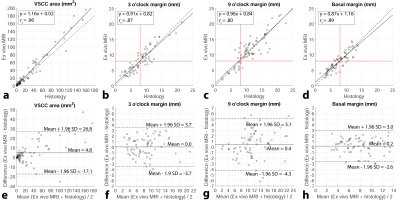
were corrected for formalin fixation induced shrinkage, whereupon linear correlation
(Spearman’s rho) and agreement (Bland-Altman analysis) with histology were
assessed. Diagnostic performance of ex vivo MRI for the localization of VSCC and
identification of margins <8 mm was investigated by calculating positive-
and negative predictive values (PPV, NPV). The Dice similarity coefficient
(DSC) was calculated to determine the spatial overlap between VSCC areas of the
non-blinded and blinded annotations.
Results
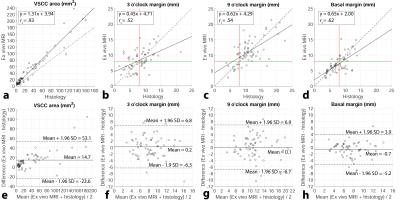
In total 153 of 159 histological slides were correlated to ex vivo MRI. In the non-blinded annotation 91 margins were identified as <8 mm, 79 were confirmed by histology (PPV = 87%). Likewise, 110 out of 124 margins were correctly identified as ≥8 mm (NPV = 89%). Ex vivo MRI measurements were significantly linearly correlated and demonstrated good agreement with histology. In the blinded annotation, absence and presence of VSCC was correctly confirmed in 73 out of 81 (NPV = 90%) and in 65 out of 72 (PPV = 90%) ex vivo MRI slices respectively. A total of 64 out of 90 margins were correctly identified as <8 mm (PPV = 71%) and 83 out of 102 margins were correctly identified as ≥8 mm (NPV = 81%). The blindly annotated VSCC area and margins were also significantly linearly correlated and demonstrated good agreement with histology. Compared to the non-blinded annotation, the linear correlation between ex vivo MRI and histology was less strong and the 95% limits of agreement in the Bland-Altman analysis were wider. The mean DSC between non-blinded and blinded annotation of ex vivo MRI was 0.73.Discussion
The non-blinded annotation of ex vivo MRI demonstrated that it is technically feasible to obtain VSCC location and surgical tumour free margin status in fresh WLE specimens with high correlation and agreement with histology using ex vivo MRI. Furthermore, the blinded annotation demonstrates the potential clinical applicability of ex vivo MRI by showing high PPV and NPV for prediction of VSCC and margins <8 mm. Limitations are the small number of patients and the fact that this was a one reader study. The ultimate goal is to obtain perioperative information on the surgical tumour free margins of WLE specimens using ex vivo MRI. In this respect the results are promising, but more research is required in the form of a randomized controlled trial.Conclusion
Accurate localization of VSCC and measurements of the surgical tumour free margins in fresh WLE specimens using ex vivo MRI is technically feasible. The high NPV and PPV for localization of VSCC and identification of margins <8 mm demonstrate clinical applicability of the technique.Acknowledgements
Ex vivo MRI was performed using equipment of the Preclinical Imaging Centre (PRIME) within the Radboud University Medical Center, Nijmegen, the Netherlands.References
1. Ansink A, van der Velden J. Surgical interventions for early squamous cell carcinoma of the vulva. Cochrane Database Syst Rev. 2000;(2):CD002036.
2. de Hullu JA, van der Zee AGJ. Surgery and radiotherapy in vulvar cancer. Crit Rev Oncol Hematol. 2006;60(1):38–58.
3. Heaps JM, Fu YS, Montz FJ, Hacker NF, Berek JS. Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol. 1990;38(3):309–14.
4. de Hullu JA, Hollema H, Lolkema S, et al. Vulvar carcinoma: The price of less radical surgery. Cancer. 2002;95(11):2331–8.
5. Chan JK, Sugiyama V, Pham H, et al. Margin distance and other clinico-pathologic prognostic factors in vulvar carcinoma: A multivariate analysis. Gynecol Oncol. 2007;104:636–41.
6. Heidkamp J, Zusterzeel P, Veltien A, Maat A, Van Engen-Van Grunsven I, Fütterer J. Ex vivo MRI evaluation of vulvar cancer to predict resection margins in fresh wide local excision specimens: a pilot study (0101). In: Proc Intl Soc Mag Reson Med 25. 2017.
Figures