1167
Markerless real-time motion correction for T1- and T2-weighted neuroanatomical MRI1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 2Department of Radiology, Boston Children's Hospital, Harvard Medical School, Boston, MA, United States, 3Fetal-Neonatal Neuroimaging and Developmental Science Center, Boston Children's Hospital, Boston, MA, United States, 4Computer Science and Artificial Intelligence Laboratory, Massachusetts Institute of Technology, Cambridge, MA, United States, 5Radiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
This study investigates real-time head motion correction using markerless tracking of the subject's face to mitigate the major problems caused by patient movement in clinical and research MRI. The effects of motion include repeat scanning, impaired clinical diagnosis, need for sedation or anesthesia, and biased research results. Markerless tracking and correction is appealing because it could offer minimal disruption to the MRI workflow, sequence independence, and high-frequency motion estimation. Real-time correction substantially improved T2-SPACE and MPRAGE image quality in scans with intentional motion, compared to uncorrected scans. Cortical surface reconstructions, brain structure volumes, and cortical thickness estimated from the motion-corrected MPRAGE scan showed good correspondence with the gold standard scans without intentional movement. Markerless real-time correction is a promising approach to reduce the effects of motion in neuro MRI.
Target Audience
Researchers and clinicians interested in using markerless
tracking systems to correct artifacts caused by head motion during MRI scans.Introduction
Motion is widely recognised as a major problem in MRI, with consequences including need for sedation or anesthesia in clinical studies, wasted time and money for repeat scanning, reduced diagnostic image quality, and biased research results, particularly when comparing groups who moved differentially during scanning1-3. In neuroimaging, high-resolution 3D-encoded scans lasting on the order of minutes are particularly susceptible to complicated motion artifacts. Retrospective k-space corrections and real-time modifications of the sequence both require measurement of motion during the scan, and corrections using MR-based “navigator” methods4-8 and tracking with external equipment9 and MR markers10 have been demonstrated. External camera and marker systems can estimate motion and update the sequence with high sampling rate, but the requirement to attach a marker to the subject’s head or face can be both prohibitive and an imperfect measure of head motion.
This study investigates markerless head motion tracking for real-time motion correction11,12 in 3D-encoded T2-SPACE and MPRAGE sequences. The high-frequency motion estimates provided by the independent tracking system allow field-of-view (FOV) corrections within the image encoding (echo-train) of each TR period.
Methods
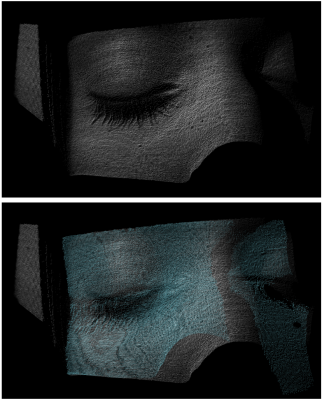
The “Tracoline” TCL3.01 markerless tracking system (TracInnovations, Copenhagen, Denmark) was used to estimate head motion11,12. The Tracoline system uses a structured light source and an optical camera attached to the scanner table to reconstruct a 3D “point cloud” model of the subject’s face, specifically around the nose and the eyes. These point clouds are registered to an initial reference point cloud to provide up to 30 motion estimates per second (see examples in Fig. 1). The updates to the scanner coordinates were calibrated within the Tracoline software by matching a point cloud reconstruction to the surface of an MPRAGE scan12.
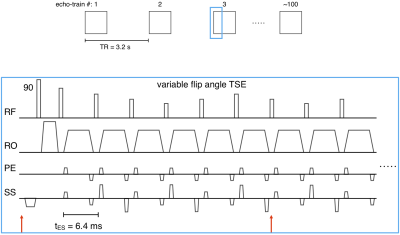
3D-encoded T2-SPACE and MPRAGE sequences were modified to apply transformations to the imaging FOV using motion estimates from the Tracoline system. The FOV was updated before the start of each echo-train, and every 6 lines of k-space thereafter until the end of each echo-train (see T2-SPACE sequence diagrams in Fig. 2). The sequence instructions were written 15ms in advance of their execution.
Data were acquired on a 3T Siemens Prisma scanner with a 64-channel head coil. T2-SPACE and MPRAGE protocols with FOV=256x256mm2, matrix=256x256, 176 1mm sagittal slices, in-plane GRAPPA R=2 were acquired. The 5:34min T2-SPACE used the following parameters: TR=3200ms; TE=565ms; bandwidth=241Hz/px; echo spacing=6.28ms; echo-train duration=1187ms; turbo factor=200. The 6:09min MPRAGE used the following parameters: TR=2500ms; TE=3.3ms; TI = 1070ms; bandwidth=240Hz/px; echo spacing=8ms; turbo factor=176.
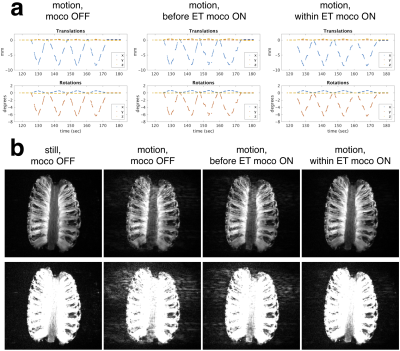
A pineapple was scanned to compare applying FOV corrections within the echo-train versus only applying a FOV correction before each echo-train, which is the same correction rate used in previous navigator techniques5,7 (i.e. once-per-TR). The pineapple was moved continuously for a ~50s period (2 minutes into the scan) in the following scans: 1) no motion correction; 2) motion correction before each echo-train (once-per-TR); 3) motion correction before each echo-train, and every 6 lines within the echo-train.
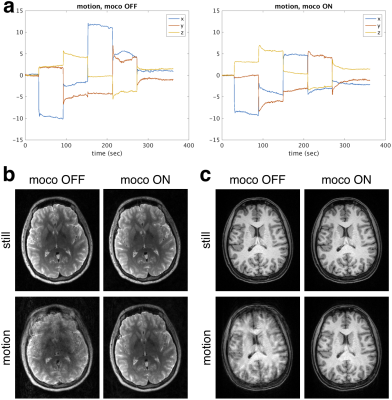
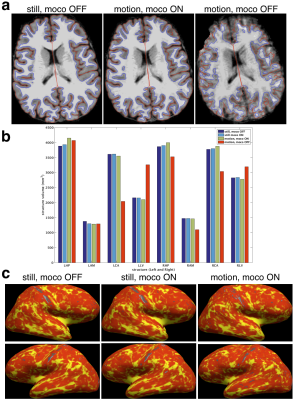
A healthy volunteer was scanned in accordance with Institutional Review Board guidelines. The volunteer was instructed to move five times at 1 minute intervals in a repeatable fashion during scans with real-time motion correction (updates every 6 lines within echo-train) and without. For comparison, images were also acquired during two scans with and without real-time motion correction when the volunteer did not move intentionally. Morphometry analysis on the MPRAGE images with FreeSurfer13,14 was performed to assess cortical surface reconstructions and structure volumes. Cortical thickness analysis was performed in a common template space15 for the two still scans and the movement scan with motion correction.
Results
The phantom results in Fig. 3 show that the within echo-train correction reduces ghosting compared with only updating before the echo-train. The reductions in T2-SPACE and MPRAGE motion artifacts with real-time markerless correction and the motion measured by the markerless tracking during the two motion scans are shown in Fig. 4 with morphometry results in Fig. 5.Discussion
The markerless real-time motion correction substantially improved in-vivo image quality and the reliability of morphometry results. The controlled phantom experiment suggests that within echo-train correction has benefits over once-per-TR correction. Furthermore, the independent markerless tracking does not have the requirement for “dead time” within the sequence to insert navigator acquisitions. Investigating the use of reacquisition for TRs corrupted by substantial motion5,7 and comparison with retrospective corrections8 will be the subject of future work.Conclusion
Markerless real-time motion correction using tracking of the subject’s face is a promising approach to reduce artifacts in neuro MRI studies.Acknowledgements
We would like to acknowledge technical support from TracInnovations and the following funding sources: NICHD R01HD093578, NICHD R01HD085813, NIMH R44MH086984, NIDA U24DA041123, NIA R21AG046657, NICHD R01HD071664.References
[1] Andre et al. J Am Coll Radiol 12:689–695, 2015.
[2] Reuter et al. NImg 107:107-115, 2015.
[3] Yendiki et al. NImg 88:79-90, 2014.
[4] van der Kouwe et al. MRM 56(5):1019-1032, 2006.
[5] White et al. MRM 63(1):91–105, 2010.
[6] Hess et al. MRM 66(2):314–323, 2011.
[7] Tisdall et al. MRM 68(2):389–399, 2012.
[8] Gallichan et al. MRM 75(3):1030–1039, 2016.
[9] Zaitsev et al. NImg 31(3):1038–1050, 2006.
[10] Ooi et al. MRM 62(4):943-954, 2009.
[11] Olesen et al. ISMRM 2014, p1303.
[12] Benjaminsen et al. ISMRM 2016, p1860.
[13] Dale et al. NImg 9:179-194, 1999.
[14] Fischl et al. NImg 9:195-207, 1999.
[15] Reuter et al. NImg 61:1402-1418, 2012.
Figures