1166
Radiomics subtyping improves 5-year disease free survival prediction beyond key clinical and radiological characteristics in patients with locally advanced rectal cancer (LARC)Ke Nie1, Peng Hu2, Fei Peng3, Tingyu Mao4, Xiao Wang1, Ning Yue1, and Jihong Sun2
1Department of Radiation Oncology, Rutgers-The State University of New Jersey, New Brunswick, NJ, United States, 2Department of Radiological Sciences, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China, 3Institute of Translational Medicine,Zhejiang University, Hangzhou, China, 4Department of Electrical Engineering, Columbia University, New York, NY, United States
Synopsis
In this study, we have identified an 11-feature set from a large panel of radiomics signature (5248 features from pre-operative T1w, T2w, DCE and DWI images) that allows prediction of 5-year disease free survival of locally advanced rectal cancer patients underwent surgical resection. The selected radiomics signature demonstrates improved performance compared with that of established clinical and radiological risk models. The results were tested and validated on a consecutive 165-patient cohort with an average of 54±20 months follow-ups.
Purpose
To evaluate whether MRI based radiomics signatures allow prediction of survival and stratification of locally advanced rectal cancer (LARC) patients with improved accuracy compared to that of established clinical and radiologic risk models.Methods
We conducted a retrospective review of a prospectively gathered database of patients LARC who underwent surgical resection between 01/2009 to 03/2012. In total, 222 consecutive cases were identified (104 females; mean age 63±12 years old, range 18-88 yo) with a mean follow-up of 54±20 months (range 5 to 98 months). Recorded data included age, sex, the endoscopic and radiological investigations, the nature of surgical procedures, the presence or occurrence of synchronous and metachronous metastatic disease, TNM stages, CEA levels, height of the distal tumor margin above the anal verge, circumferential resection margin involvement, and pathological staging. In order to ensure the accuracy of data, all histological and radiological data were reviewed by two experienced radiologists. The final clinical endpoint is the long-term disease-free survival (DFS), which is defined as the time from the date of surgical resection until the date of relapse. All patients received pre-operative multi-parametric MRI for local tumor and distant metastases staging. The MRI was done on a 3.0 Tesla scanner (GE Signa HDxt) using a phased-array body coil. The imaging protocol included T1w, T2w, DCE-MRI acquired using a spoiled gradient echo sequence (LAVA), and DWI acquired using a single-shot echo planar imaging sequence (SSEPI) with two b-factors of 0 and 800 s/mm2. 5248 radiomics features were extracted for each patient, including morphological, first-order histogram-based, second-order textural-based and higher-order wavelet-based features. A total of 165 patients with complete clinical data and radiological images were included for analysis. Patients were randomly assigned to the training [n=114] or validating [n=51] set with an allocated 7:3 ratios. Least absolute shrinkage and selection operator (LASSO) regression was applied to select the best optimal feature set in the training dataset. The robustness of feature selection was trained with 100 times of 10-fold-cross-validation. Then a radiomics score was computed for each patient through a linear combination of selected features weighted by their respective coefficient. To demonstrate the incremental value of the radiomics signature to the established clinical and radiological risk factors, ROC analyses of conventional clinical/radiological/pathological factors, radiomics factors and combined all information were conducted in both training and validating sets. In addition, a nomogram incorporated the radiomics signature and clinical/radiological/pathological features based on the multivariate Cox analysis were provided to assess survival time or survival probability. The patients were further classified into high-risk or low-risk groups and the potential association of all combined signatures with DFS were assessed using Kaplan-Meier survival analysis.Results
LASSO analysis allowed an optimal selection of 11 radiomics signatures as shown in Figure 1. The gray-level co-occurrence matrix based parameter “standard deviation of energy” derived from a wavelet transformation of LAVA, had the highest importance score related to worse survival. The radiomics signatures were significantly associated with DFS, shown in the heatmap of Figure 2. The results were further validated successfully in the validation set for 5-year DFS stratification with ROC curves shown in Figure 3. With selected radiomics features, the AUC outperformed than the conventional clinical-radiological-pathological models. In addition, radiomics information demonstrated its incremental value in improving the 5-year DFS prediction in both training and validating set. A nomogram integrated the radiomics signature and the clinical data were developed for both predictions of 3-/5-year DFS (C-index of 0.776) and overall survival months (C-index of 0.706) for each individual patient as shown in Figure 4. The patients were separated into high- and low-risk groups using Youden’s index and the corresponding Kaplan-Meier survival curves were shown in Figure 5.Conclusion
To our best knowledge, the association of radiomics features with survival of patients diagnosed with locally advanced rectal cancer has not been evaluated before. This is probably the first and also the largest study combining both clinic-radiological-pathological risk factors, and radiomics signatures for prediction of long-term disease free survival after surgical resection. Qe have built and validated an effective algorithm for survival classification and demonstrate improved performance with non-invasive radiomics profiling compared with that of established clinical and radiologic risk models. In addition, a nomogram that incorporates the radiomics signature and independent clinic-pathologic risk factors was built which might provide clinician an intuitive tool for evaluating clinical outcomes in patients with LARC.Acknowledgements
This study is supported in part by Rutgers-RBHS precision medicine pilot grant, The Rutgers-Cancer Institute of New Jersey P30CA072720References
No reference found.Figures
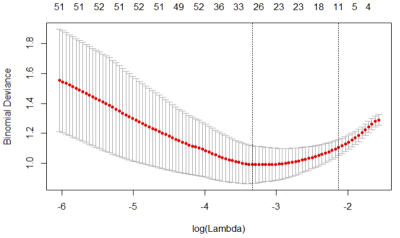
Figure 1. Radiomics feature selection using the least absolute
shrinkage and selection operator (LASSO) logistic regression model. Identification
of the optimal penalization coefficient lambda using 10-fold cross-validation
resulted in an eleven-radiomics-feature set
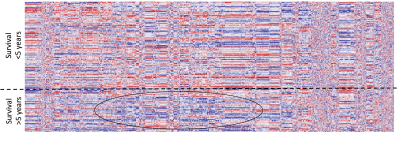
Figure 2.
Radiomics heatmap. Unsupervised clustering of patients with LARC is
shown on the y-axis, and radiomics feature expression is shown on the x-axis,
revealing clusters of patients with similar radiomic expression patterns.
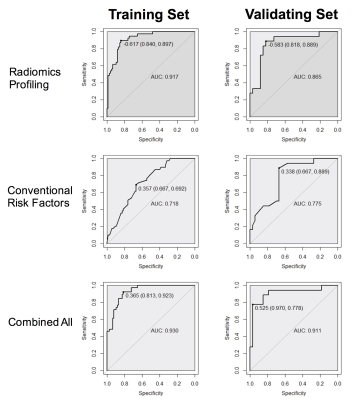
Figure 3. The ROC
analyses of (1) 11 selected radiomics signatures, (2) conventional risk factors
including clinical, radiological and pathological features, and (3) combined
radiomics and conventional risk factors in both training and validating
datasets.
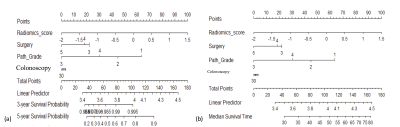
Figure 4. Use of the constructed nomogram with combined radiomics score and
clinical-radiologic-pathological factors to estimate (a) the 3-year and 5-year
disease free survival, and (b) the overall survival months for each individual patient.
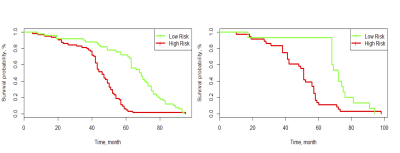
Figure 5. Graphs show results of Kaplan-Meier survival analyses according to the combined
radiomics signature and clinical risk factors for patients in the training data
set (left) and those in the validation data set (right). A significant
association of the selected radiomics and clinical signature with the DFS was
shown in the training data set, which was then confirmed in the validation data
set.