1161
Susceptibility contrast-MRI predicts response to the vascular endothelial growth factor receptor inhibitor cediranib in the Th-MYCN model of neuroblastoma1Division of Radiotherapy and Imaging, Institute of Cancer Research, London, United Kingdom, 2Division of Clinical Studies, Institute of Cancer Research, London, United Kingdom, 3Division of Molecular Pathology, Institute of Cancer Research, London, United Kingdom
Synopsis
In this study we demonstrate that fractional blood volume, measured by susceptibility contrast-MRI, predicts response to the vascular endothelial growth factor receptor inhibitor cediranib in the Th-MYCN genetically-engineered mouse model of neuroblastoma.
Introduction / Purpose
In neuroblastoma, a childhood cancer of the developing nervous system, amplification of the proto-oncogene MYCN correlates with enhanced angiogenesis and poor survival. The hyper-vascular nature of high-risk disease prompted the current evaluation of numerous small molecule inhibitors of the VEGF signaling pathway in early-phase pediatric clinical trials against neuroblastoma.1
Noninvasive MRI biomarkers are of particular importance for the delivery of precision medicine to children with cancer, as the difficulty of obtaining post-surgical biopsies in the pediatric population often precludes the assessment of conventional pharmacodynamic biomarkers of response to treatment.2 However, MRI biomarkers require robust histopathological cross-validation before they can routinely be deployed in clinical trials, a process that can be meaningfully done in animal models of cancer.3
We have previously demonstrated that cediranib is a potent inhibitor of VEGFR signalling in the Th-MYCN genetically-engineered model of neuroblastoma, which faithfully recapitulates the major patho-physiological and radiologic features of the childhood disease, resulting in a rapid reduction in tumor perfusion 24h after treatment.4,5
Here we evaluate and, using whole-slide high-resolution digitised pathology, validate that fractional blood volume (fBV) quantified by susceptibility contrast (SC)-MRI i) provides a robust biomarker of vascular perfusion and its therapeutic modulation and ii) predicts long-term tumor volume response to treatment with cediranib in neuroblastomas arising in the Th-MYCN model.
Methods
All experiments were performed in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986 and the United Kingdom National Cancer Research Institute guidelines for the welfare of animals in cancer research.6
Th-MYCN mice with spontaneously arising abdominal neuroblastoma were identified by palpation. On day 1, mice were treated daily with 6mg/kg cediranib (AstraZeneca) p.o. (n=18) or vehicle (n=7). T2-weighted MRI and SC-MRI were performed on day0/day 2 (24h after treatment) in n=7 mice both in treated and vehicle cohort and additionally on day 7 for an additional n=11 mice in the treated cohort.
MRI studies were performed on a 7T Bruker MicroImaging system using a 3cm birdcage volume coil. T2-weighted axial images were used for determining tumor volumes and, following optimization of the local field homogeneity (Fastmap), to plan transverse relaxation rate R2* measurement.7 Multi gradient echo images (FOV 3x3cm2, 128x128 phase encoding steps, TE=6-30ms, 8 TEs, TR=200ms, NEX=8) were acquired prior to and 3 min after i.v. injection of ultrasmall superparamagnetic iron oxide (USPIO) particles (P904, 150μmolFe/kg, Guerbet) and fBV(%) derived voxelwise as previously described.8
Histology. Guided by T2-weighted anatomical images, tumors were carefully excised and orientated for subsequent histopathological processing. Formalin-fixed and paraffin embedded tumors were cut in 3μm sections and stained with hematoxylin and eosin (H&E). IHC detection of the vascular endothelial cell marker endomucin (Cd34) was used to assess microvessel density.
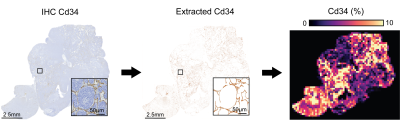
Comparison of fBV and Cd34 parametric maps. Cd34 was extracted form digitized whole-slide sections (20x magnification, Hamamatsu NanoZoomer XR, 97% accuracy based on independent annotation of stained/non-stained points (688/734, n=9 samples)) showing good spatial correspondence with T2-weighted images based on the visual assessment of anatomical landmarks (tumor shape, position of abdominal vessels, lymph nodes and kidneys). Cd34 parametric maps were generated as described in Fig 1.
Results
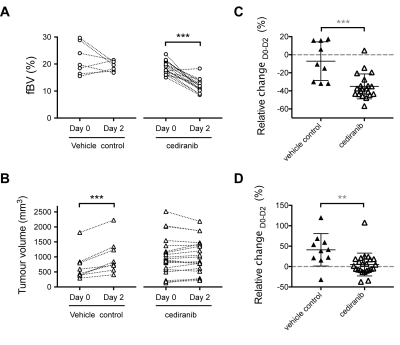
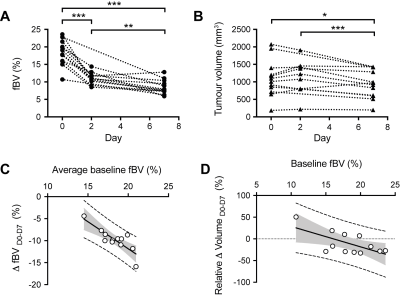
Cediranib induced a rapid reduction in tumor median fBV and significantly inhibited tumor growth (Fig 2). Six days of continuous daily treatment with cediranib caused a further significant reduction in fBV and anti-tumor activity (Fig 3). The average baseline fBV strongly correlated with the cediranib-induced change in fBV after 7 days treatment. Baseline fBV correlated with cediranib-induced tumor volumetric response at day 7.
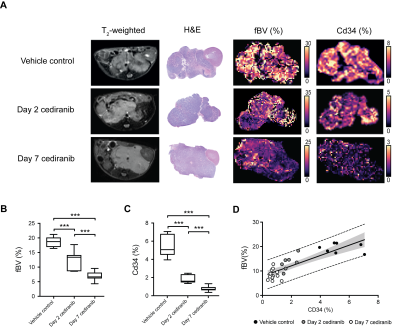
Regional variations in fBV positively reflected variations in Cd34 staining in their respective parametric maps (Fig 4). Quantification of Cd34 fraction area corroborated the cediranib-mediated fBV reduction measured in vivo. Median tumor fBV strongly correlated with Cd34 fraction area.
Discussion
This MRI/digital histology comparison study validates fBV as a robust biomarker of vascular perfusion and its ability to inform on therapeutic modulation. In addition, fBV provides a predictive biomarker of vascular response, similar to that recently reported with another VEGFR inhibitor against renal cancer xenografts8, and, more interestingly, a predictive biomarker of tumor response to cediranib in the Th-MYCN model of neuroblastoma.
SC-MRI provides an attractive steady-state alternative for the pediatric population in which dynamic contrast enhanced-MRI protocols can be challenging to perform. Recent clinical studies have highlighted the safety off-label use of the USPIO preparation Ferumoxytol for MRI in children and young adults9, supporting further investigation of SC-MRI in early phase pediatric clinical trials of vascular–targeted or vascular-modulating therapies against neuroblastoma.
Conclusion
Baseline tumor fBV is a predictive biomarker of response to the potent VEGFR inhibitor cediranib in the Th-MYCN model of neuroblastoma.Acknowledgements
We acknowledge support from a Children with Cancer UK Research Fellowship, Rosetrees Trust Grant A1091, The Institute of Cancer Research Cancer Research UK Cancer Imaging Centre, in association with the MRC and Department of Health (England) grant C1060/A16464, NHS funding to the NIHR Biomedical Research Centre, a Cancer Research UK Programme Grant (C18339).References
1 Roy Choudhury, S., Karmakar, S., Banik, N. L. et al. Targeting Angiogenesis for Controlling Neuroblastoma. Journal of Oncology. 2012; 2012(15).
2 Daldrup-Link, H. E., Sammet, C., Hernanz-Schulman, M. et al. White Paper on P4 Concepts for Pediatric Imaging. J Am Coll Radiol. 2016; 13(5): 590-597 e592.
3 O'Connor, J. P., Aboagye, E. O., Adams, J. E. et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017; 14(3): 169-186.
4 Chesler, L. & Weiss, W. A. Genetically engineered murine models--contribution to our understanding of the genetics, molecular pathology and therapeutic targeting of neuroblastoma. Semin Cancer Biol. 2011; 21(4): 245-255.
5 Jamin, Y., Tucker, E. R., Poon, E. et al. Evaluation of clinically translatable MR imaging biomarkers of therapeutic response in the TH-MYCN transgenic mouse model of neuroblastoma. Radiology. 2013; 266(1): 130-140.
6 Workman, P., Aboagye, E., Balkwill, F. et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010; 102(11): 1555-1577.
7 Jamin, Y., Glass, L., Hallsworth, A. et al. Intrinsic susceptibility MRI identifies tumors with ALKF1174L mutation in genetically-engineered murine models of high-risk neuroblastoma. PLoS One. 2014; 9(3): e92886.
8 Robinson, S. P., Boult, J. K. R., Vasudev, N. S. et al. Monitoring the Vascular Response and Resistance to Sunitinib in Renal Cell Carcinoma In Vivo with Susceptibility Contrast MRI. Cancer Res. 2017; 77(15): 4127-4134.
9. Muehe, A.M., Feng, D., von Eyben, R., et al. Safety Report of Ferumoxytol for Magnetic Resonance Imaging in Children and Young Adults. Invest Radiol 51, 221-227 (2016).
10. Schindelin, J., Arganda-Carreras, I., Frise, E., et al. Fiji: an open-source platform for biological-image analysis. Nature methods 9, 676-682 (2012).
11. Schindelin, J., Rueden, C.T., Hiner, M.C., et al. The ImageJ ecosystem: An open platform for biomedical image analysis. Molecular reproduction and development 82, 518-529 (2015).
Figures