1158
Multi-modality molecular imaging with hyperpolarized [1-13C]pyruvate MRSI and 18FDG-PET of early tumor response to a novel TRAIL agonist (MEDI3039)1CRUK Cambridge Institute, University of Cambridge, Cambridge, United Kingdom, 2CRUK Beatson Institute, Glasgow, United Kingdom, 3Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom
Synopsis
This study compared the capability of 3D magnetic resonance spectroscopic imaging (MRSI) of hyperpolarized [1-13C]pyruvate metabolism and 18FDG-PET to detect early responses to a TRAIL agonist (Medimmune MEDI3039) in Colo205 colorectal cancer and MDA-MB-231 triple-negative breast cancer xenograft models expressing luciferase and mStrawberry fluorescent protein. 24 hours after treatment, bioluminescence and hyperpolarized lactate/pyruvate ratio decreased significantly in all treated animals, preceding decreases in tumor volume. Conversely, 18FDG-PET did not detect treatment response. This suggests that for some tumors hyperpolarized [1-13C]pyruvate may be an improvement on 18FDG-PET and RECIST for the early detection of treatment response.
Introduction
We compared the capability of PET imaging of 2-deoxy-2-(18F)fluoro-D-glucose (18FDG) and 3D magnetic resonance spectroscopic imaging (MRSI) of hyperpolarized [1-13C]pyruvate metabolism to detect early responses to a TRAIL agonist (Medimmune MEDI3039) in Colo205 colorectal cancer and MDA-MB-231 triple-negative breast cancer xenograft models.
Methods
Colo205 and MDA-MB-231 cells (ATCC) were transfected with luciferase and mStrawberry reporter genes. 5 ×106 cells were implanted subcutaneously into the right flank of BALB/c nude mice and tumors grown until they were 0.8 ± 0.4 cm3. All mice underwent bioluminescence and fluorescence imaging, hyperpolarized [1-13C]pyruvate MRSI and 18FDG-PET imaging in a single 2 hour imaging session repeated before and 24 hours after treatment with MEDI3039. Bioluminescence images were acquired 5 min after injection of 150 mg/kg D-luciferin. 18FDG (13.08±1.7 MBq) and hyperpolarized [1-13C]pyruvate (15 ml/kg 75mM) were injected intravenously. A spiral 3D spectral-spatial pulse sequence was used to excite alternately the [1-13C]pyruvate and [1-13C]lactate resonances. Images of each metabolite had a spatial resolution of 1.25 ×1.25 × 2.5 mm and a temporal resolution of 1 s.1 Following hyperpolarized MRI a 15 min static PET acquisition began at 90 min post-18FDG administration using a NanoPET/CT (Mediso). Images were analyzed and co-registered in VivoQuant (InviCRO) and Matlab (Mathworks). Ex vivo analysis of tumors included 1H and 13C NMR of tumor extracts following [1,6-13C]glucose infusion, western blotting, histology, immunohistochemistry and FACS sorting of tumors following 18FDG administration.
Results
In Colo205 tumors, treatment with MEDI3039 resulted in a mean decrease of 71±31% in tumor bioluminescence (P = 0.005). This was attributed, at least in part, to a mean decrease of 61% in ATP measurements made ex vivo (P = 0.0008). Hyperpolarized MRSI was completed in 10 animals, six undergoing treatment with MEDI3039 and four controls. A decrease in the lactate/pyruvate ratio was observed in all MEDI3039-treated tumors, the mean pre-treatment ratio of 2.4±0.5 decreasing to 1.3±0.3 after treatment (P = 0.01). This was consistent with significant increases in TUNEL and cleaved caspase-3 (CC3) staining of tumor sections of 137% and 168%, respectively (P = 0.007 and 0.0002, respectively). 18FDG-PET was completed in 13 animals (7 treated and 6 controls) and did not detect a response to MEDI3039 treatment, with a mean difference in SUVmax of 0.03 (P = 0.8) and SUVmean of 0.2 (P = 0.6). mStrawberry fluorescence intensity and tumor volume also did not change 24 hours after treatment (P = 0.22 and 0.35, respectively). Across all modalities control treatment did not result in significant changes in the measured signals. Similar observations were made in MDA-MB-231 tumors, with bioluminescence and hyperpolarized MRSI detecting response in all seven tumors treated with MEDI3039. Tumor bioluminescence decreased by 60±29% and the hyperpolarized lactate/pyruvate ratio decreased from 2.2±0.5 to 1.4±0.3. There was no significant change in FDG SUVmax after treatment (P = 0.09). 18FDG autoradiography of Colo205 tumors confirmed the in vivo PET findings that tumour FDG uptake was unchanged after treatment. Western blot analysis showed a significant decrease in expression of GLUT1 and GLUT3 after treatment. Following [1,6-13C]glucose infusions into Colo205 tumor-bearing mice, ex vivo 13C NMR measurements on tumor extracts showed similar glucose labelling in tumors before and after treatment but treatment resulted in a large decrease in all downstream metabolites.
Discussion
Response to treatment with a novel TRAIL agonist, MEDI3039, was detected in Colo205 and MDA-MB-231 tumors just 24 hours after treatment using both BLI and 13C MRSI with hyperpolarized [1-13C]pyruvate, which preceded changes in tumor volume. Conversely, 18FDG-PET and mStrawberry fluorescence did not detect response at this time. Ex vivo analysis of Colo205 tumors suggest that despite a decrease in tumor glycolysis, after treatment with MEDI3039 it is still possible for glucose and 18FDG to accumulate in the tumor, resulting in 18FDG-PET being unable to detect treatment response.
Conclusions
This result suggests that for some tumors hyperpolarized [1-13C]pyruvate may be an improvement on 18FDG-PET and RECIST for the early detection of treatment response.
Acknowledgements
No acknowledgement found.References
1. Wang J, Wright AJ, Hu DE, Hesketh R, Brindle KM. Single shot three-dimensional pulse sequence for hyperpolarized 13C MRI. Magn Reson Med. 2016, Feb 24. Doi: 10.1002/mrm.26168. [Epub 2016 Feb 24].Figures
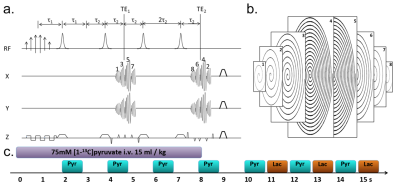
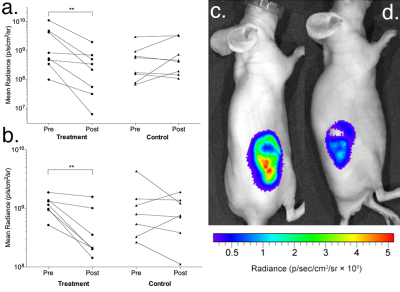
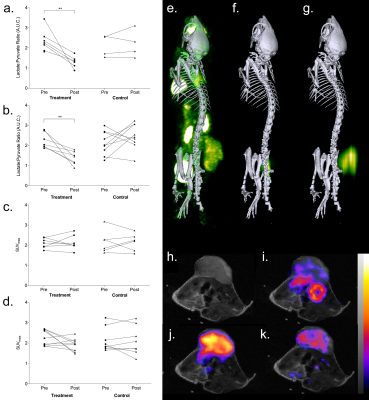
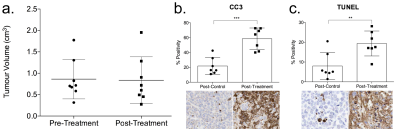
Figure 4. (a) Changes in Colo205 tumor volume 24 hours after MEDI3039 treatment. Tumor volume was measured using the T2-weighted MRI image and there was no change in volume detected after treatment (P = 0.35). At the same time point, ex vivo immunohistochemistry showed that after treatment there was significant activation of apoptosis resulting in an increase of (b) CC3 positivity from 22±12 to 59±14% (P = 0.0002) and (c) terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) from 8±6.7 to 19±6.3% (P = 0.007).