1155
A Feasibility Study of Radiation Therapy Dose Escalation Guided by Spectroscopic Magnetic Resonance Imaging in Patients with GlioblastomaSaumya S. Gurbani1,2, Eric Mellon3, Brent D. Weinberg2, Eduard Schreibmann1, Andrew A. Maudlsey4, Sulaiman Sheriff4, Peter B. Barker5, Lawrence Kleinberg6, Lee A. D. Cooper7, Hui-Kuo Shu1, and Hyunsuk Shim1,2
1Department of Radiation Oncology, Winship Cancer Institute of Emory University, Atlanta, GA, United States, 2Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, GA, United States, 3Department of Radiation Oncology, University of Miami Miller School of Medicine, Miami, FL, United States, 4Department of Radiology, University of Miami Miller School of Medicine, Miami, FL, United States, 5Department of Radiology and Radiological Science, The Johns Hopkins University, Baltimore, MD, United States, 6Department of Radiation Oncology, The Johns Hopkins University, Baltimore, MD, United States, 7Department of Biomedical Informatics, Emory University School of Medicine, Atlanta, GA, United States
Synopsis
Glioblastoma (GBM) is a grade IV primary brain tumor with poor outcomes despite surgical resection, chemotherapy, and radiation. Often, disease will recur in regions in the penumbra of the treatment volume, hypothesized to occur because anatomic MRI does not fully capture neoplastic infiltration. Spectroscopic magnetic resonance imaging (sMRI) enables in vivo whole-brain analysis of metabolic activity, and has been shown to sensitively and specifically identify regions of non-enhancing, infiltrating tumor. We present an ongoing prospective clinical study to target metabolically active tumor identified by sMRI for a radiation boost, with the aim of improving outcome in patients with GBM.
Introduction
Glioblastoma (GBM) is a malignant primary adult brain tumor with an annual incidence of 7,000 cases in the United States (1). Current treatment recommendations include maximal safe resection followed by concurrent radiation therapy (RT) and temozolomide chemotherapy (2). High dose RT is targeted using T1-weighted contrast-enhanced (CE-T1w) MRI, in which areas of enhancement represent areas of tumor with leaky neovasculature. Lower dose RT is targeted using T2-weighted fluid-attenuation inversion recovery (FLAIR) MRI, in which areas of hyperintensity include a combination of infiltrative tumor, inflammation, and vasogenic edema (3). Despite aggressive treatment, median survival remains 15 months (4, 5). Often, disease recurs within the penumbra of the treatment volume, hypothesized to occur because CE-T1w and FLAIR do not capture the entirety of tumor, including areas of proliferation beyond the tumor core. Spectroscopic magnetic resonance imaging (sMRI) is an evolution of MR spectroscopy that enables 3D whole-brain volumes of metabolic activity to be obtained in vivo without any exogenous contrast agents (6, 7) (Fig. 1). We have previously shown that the ratio of choline to N-acetylaspartate (Cho/NAA) sensitively and specifically identifies tumor proliferation; regions of metabolic activity expressing a Cho/NAA ratio more than two times that of normal appearing white matter are be prone to recurrence if left untreated (8). Despite its potential to improve disease control, clinical adoption of sMRI has been limited by difficulty incorporating it into clinical display software without manual workarounds (9) and artifacts due to magnetic field inhomogeneities (10-12). We present a cloud software platform designed specifically to incorporate sMRI into the RT planning workflow. and demonstrate its feasibility in guiding RT based on elevated Cho/NAA in a multisite clinical study.Methods
We built the Brain Imaging Collaboration Suite (BrICS), a web-based software designed to integrate sMRI with clinical 3D MRI volumes, enabling physicians to evaluate metabolic activity, review underlying spectra, and delineate target volumes for RT planning (Fig. 2a). Data from spectroscopy software, such as the Metabolite Imaging and Data Analysis Suite (MIDAS), are automatically registered via affine transformation with clinical DICOM volumes (CE-T1w MRI, FLAIR MRI). BrICS encapsulates algorithmic modules, such as automated contouring of residual contrast enhancement, which reduces user bias and improve reproducibility across patients (Fig. 2b). While MIDAS eliminates spectra that have broadened peak linewidths, some artifactual spectra remain. We developed a convolutional neural network, trained on MR experts’ quality classifications of spectral artifacts (poor/unacceptable spectra), to perform additional filtering (Fig. 2c). It takes as input unfitted spectra and outputs a classification of “good” or “poor” quality for each voxel; poor quality spectra are then filtered out. To assess feasibility of using BrICS in the multisite settings, patients were enrolled from three institutions – Emory, Johns Hopkins, and U. Miami. Each underwent a pre-RT sMRI and regions of the brain with a two-fold increase in Cho/NAA were automatically contoured. Data were reviewed collaboratively in BrICS by two spectroscopists, who assessed raw and fitted spectra, and a neuroradiologist; voxels with poor quality spectra were eliminated from the target volume. Two radiation oncologists then made final edits and validated volume for RT treatment. All user edits were tracked in a digital “paper trail” for safety and retrospective review of the pipeline. Finally, BrICS generated DICOM RT sets that are imported into clinical LINAC stations. For this study, elevated Cho/NAA and residual contrast-enhancing lesions were targeted for boosted radiation to 75 Gy; the remaining tissue received standard-of-care therapy to 60/51 Gy (Fig. 3).Results
Two cases from this study are shown. Patient one is a 21-year-old female with a frontal GBM at post-resection. A small amount of residual contrast-enhancement (1.62 cm3) remains after surgery (Fig. 4a), though the Cho/NAA abnormality is much larger (48.21 cm3) and includes a contralateral projection through the genu of the corpus callosum (Fig. 4b). Based on these two contours, a 75 Gy boost (PTV3) was planned (Fig. 4c). Patient two is a 57-year-old male with a parieto-occipital GBM. Residual parietal enhancement (2.59 cm3) and an anterior projection of Cho/NAA (23.84 cm3) abnormality were used as the target volume for RT boost (Fig. 5). Importantly, analyses are performed on a central server, ensuring unbiased reproducibility of contours.Conclusion
We have developed a cloud platform specifically designed to enable collaborative sMRI-guided radiation therapy in the multisite setting, paving the way for future consortium trials to evaluate the efficacy of sMRI as a prognostic tool. With continued evolution of this software, the data processing pipeline, and data from future studies, sMRI moves closer to clinical use, and ultimately to improving patient outcomes.Acknowledgements
This work is funded by the following grants from the National Institutes of Health: U01 CA172027 (Shim, Barker, Shu); R01 CA214557 (Shim, Shu); and F30 CA206291 (Gurbani).References
- Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(suppl_4):iv1-iv63.
- Wen PY, Macdonald DR, Reardon DA. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010.
- Tsuchiya K, Mizutani Y. Preliminary evaluation of fluid-attenuated inversion-recovery MR in the diagnosis of intracranial tumors. Am J Neuroradiol. 1996.
- Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25(26):4127-36.
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-66.
- Law M. MR spectroscopy of brain tumors. Topics in Magnetic Resonance Imaging. 2004;15(5):291-313.
- Maudsley AA, Domenig C, Sheriff S. Reproducibility of serial whole‐brain MR Spectroscopic Imaging. NMR in Biomedicine. 2010.
- Cordova JS, Shu H-KG, Liang Z, Gurbani SS, Cooper LAD, Holder CA, et al. Whole-brain spectroscopic MRI biomarkers identify infiltrating margins in glioblastoma patients. Neuro Oncol. 2016;18(8):1180-9.
- Cordova JS, Gurbani SS, Olson JJ, Liang Z, Cooper LAD, Shu H-KG, et al. A systematic pipeline for the objective comparison of whole-brain spectroscopic MRI with histology in biopsy specimens from grade III glioma. Tomography. 2016;2(2):106-16.
- Kreis R. Issues of spectral quality in clinical 1H magnetic resonance spectroscopy and a gallery of artifacts. NMR in Biomedicine. 2004;17(6):361-81.
- Pedrosa de Barros N, McKinley R, Knecht U, Wiest R, Slotboom J. Automatic quality control in clinical (1)H MRSI of brain cancer. NMR Biomed. 2016;29(5):563-75.
- Pedrosa de Barros N, McKinley R, Wiest R, Slotboom J. Improving labeling efficiency in automatic quality control of MRSI data. Magn Reson Med. 2017.
Figures
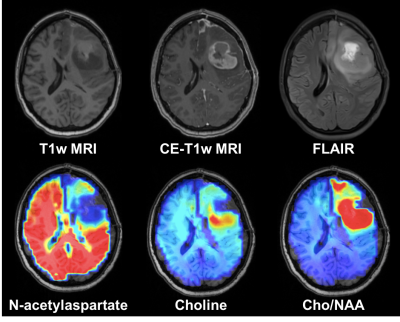
Spectroscopic
magnetic resonance imaging (sMRI) is a 3D evolution of clinical MR spectroscopy
that permits whole-brain analysis of tissue metabolism. In patients with high
grade gliomas, metabolite abnormalities such as increased choline and decreased
N-acetylaspartate can identify neoplastic infiltration beyond what is seen in on
conventional MRI.
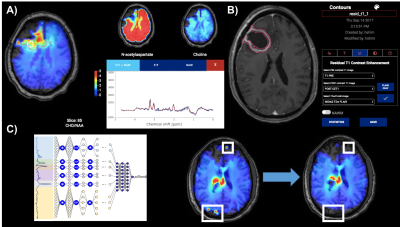
(a) BrICS is a cloud platform developed specifically for enabling radiation
therapy planning based on sMRI. Metabolite maps are fused with clinical MRI for
functional contouring, while maintaining the ability to assess underlying
spectra. (b) To identify residual enhancement, a pre-contrast T1w MRI is
histogram normalized and subtracted from a post-contrast T1w MRI; a FLAIR
hyperintensity envelope is used to restrict the region-of-interest, and
morphological filters are applied to remove islets and blood vessels. (c) To assist with artifact removal, a neural
network takes raw spectra and classifies them as real or artifactual; the
network performs whole-brain filtering in real-time.

Thirty
patients with glioblastoma will be enrolled in this trial and will undergo an
sMRI scan as part of radiation therapy (RT) planning. Regions of abnormal
metabolic activity, as defined by a Cho/NAA ratio twofold elevated to
contralateral tissue and reviewed by MR spectroscopists for accuracy, and any
residual contrast-enhancing tumor will be targeted for a RT boost to 75 Gy. The
remaining tissue will be treated with standard of care RT (60 Gy and 51 Gy).
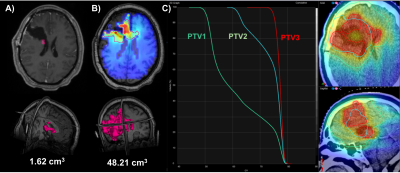
(a) A 21-year-old female with a right frontal lobe tumor underwent surgical
resection, leaving only a small residual contrast-enhancing lesion. (b) However,
an analysis of the Cho/NAA shows extension of metabolic abnormality along the
frontal lobe and even crossing into the contralateral hemisphere. (c) This
region is targeted for a radiation boost to 75 Gy (PTV3) in addition to standard
of care treatments to the resection cavity (60 Gy, PTV2), and to regions of
FLAIR abnormality (51 Gy, PTV1).
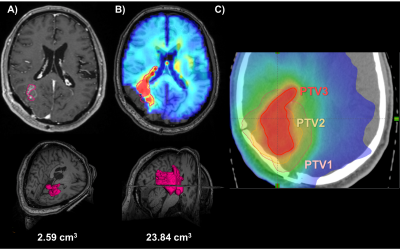
(a) A 57-year-old male with a parieto-occipital glioblastoma showed only a
small occipital residual contrast-enhancing lesion, (b) while the Cho/NAA
volume showed infiltration projection anteriorly towards the ventricles and
deep brain nuclei. (c) These two regions were targeted for RT boost to 75 Gy
(PTV3).