1152
Probing the Excitatory-Inhibitory Balance in Humans during Probabilistic Learning1Department of Neurobiology, Weizmann Institute of Science, Rehovot, Israel, 2Department of Chemical Physics, Weizmann Institute of Science, Rehovot, Israel
Synopsis
The dorsal anterior cingulate cortex (dACC) is crucial for reinforcement learning and decision-making. However, the excitatory and inhibitory mechanisms underlying these functions, governed by glutamate and GABA, are not properly understood. We used 1H-MRS to probe glutamate and GABA in the dACC during a task comprised of three conditions: discrimination, uncertainty and a null condition. A preference to higher gain option during the discrimination condition was reflected by elevated GABA levels during the uncertainty condition compared to the discrimination condition. Elevated GABA levels during the null condition predicted better behavioral-acquisition. These results indicate dACC involvement during learning of high load cognitive situation.
Introduction:
The dorsal anterior cingulate cortex (dACC) is crucial for reinforcement learning and for reward-guided decision‑making 1. However, the excitatory and inhibitory mechanisms underlying these functions, which are mainly governed by the neurotransmitters glutamate and GABA, are still not properly understood. Additionally, modulations in the excitation‑inhibition (E/I) balance, previously suggested to facilitate learning 2, 3, have not yet been demonstrated in humans. We therefore used 1H-MRS and measured GABA and Glx (glutamate + glutamine) in order to probe modulations in the E/I balance in the dACC during engagement in an instrumental probabilistic learning paradigm.Materials & Methods:
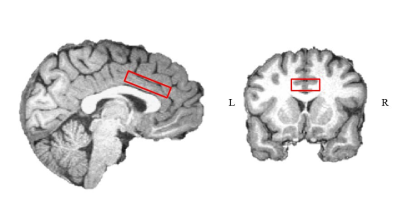
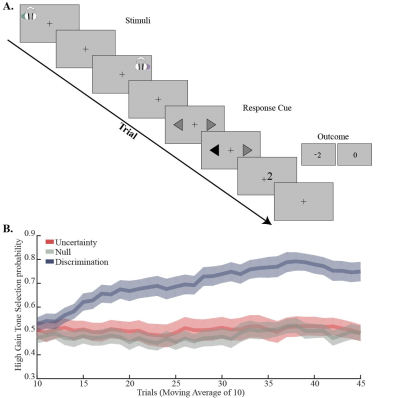
Thirty-seven right-handed healthy subjects (median age 26; 21 females) participated in the experiment. Three subjects were excluded from the experiment due to distorted signals, and additional three due to recurrent GABA and Glx outlier levels (more than 2.5 standard deviations). Participants were scanned in a 3 Tesla Tim Trio scanner (Siemens, Erlangen). Anatomical images were acquired with a 12‑channel receiver head coil (MPRAGE, TR/TE = 2300ms /2.98ms TA= 4:44 min) to enable localization of a 40×25×10 mm3 1H‑MRS voxel in the dACC (Fig. 1). In each trial during the behavioral paradigm (Fig. 2A), the subjects heard two pure-frequency tones, which were played out in succession with alternating laterality. Subsequently, a response cue appeared (arrows) and the subjects chose their preferred tone by selecting the side this tone was played to. Following the selection the arrow pointing on the preferred side was marked in black and an outcome screen appeared, presenting either a monetary reward (+2), loss (-2) or neutral feedback (0). The behavioral paradigm was assembled of three types of experimental conditions that followed each other in time and consisted of a null condition with a consistent neutral feedback; an uncertainty condition with 50/50 probability to lose or gain money; and a discrimination condition with 80/20 probability to lose or gain money. GABA and Glx were acquired throughout MEGA-PRESS (TR/TE = 2000ms /68ms). At the onset of each experimental condition block, a metabolite scan was initiated (144 averages, TA=9:57min), followed by a water reference scan (16 averages, TA=1min). Quantification was carried out using peak area integration for GABA (2.83 to 3.19 ppm) and Glx (3.56 to 3.94 ppm) and computing its ratio to the integral of the water reference signal. Macromolecular contributions to the GABA signal were not accounted for; however, we assumed they remain constant throughout the paradigm when interpreting our results.Results:
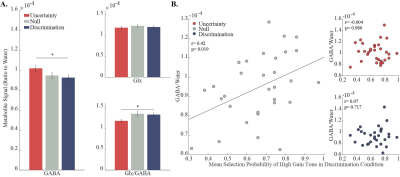
Over the course of the discrimination condition the subjects increased their preference to the tone that was associated with high gain, and by the end of this block the mean selection probability exceeded 70% (Fig. 2B). In comparison, no preference to either one of the options was observed during the two other conditions. These behavioral differences were expressed as a statistically significant elevation in GABA/Water, and a statistically significant decrease in Glx/GABA levels during the uncertainty condition compared to the discrimination condition (Fig. 3A; p<0.05, repeated measures one way ANOVA; p<0.05, post hoc Tukey-Kramer). Examination of the connection between metabolite levels and the behavioral performance revealed that higher GABA/Water levels in the null condition predicted faster (r=-0. 550, p=0.001) and better (r=0.418, p=0.019) behavioral-acquisition (Fig. 3B).Discussion & Conclusions:
The observed behavioral differences between the conditions were reflected by elevated GABA levels, which can be interpreted as increased inhibition. This increased inhibition occurs in situations that modulate dACC activity, such as uncertain rewards 4, high mental effort 5, and requirements for cognitive control 6. Therefore, presumably, during decision-making and a high cognitive load, the dACC is recruited and produces increased inhibition in order to achieve efficient and better performance (Fig. 3A). Similarly, individual differences in inhibition levels in the dACC might indicate an individual level of experienced mental effort, uncertainty or a need for cognitive control (Fig. 3B). In a neutral situation, such as the null condition, the influences of external cognitive aspects of learning such as reward incentives are eliminated and the underlying motivational or attentional processes are exposed. Therefore, the correlation between inhibition and performance in the dACC might reflect the connection between mental-effort/cognitive-control and the motivational processes that take place during learning and lead to a better performance 7-9. The paradigm presented herein will hopefully broaden our understanding of inhibitory and excitatory mechanisms involved in learning and in other cognitive conditions.Acknowledgements
No acknowledgement found.References
- Kennerley, S.W., et al., Optimal decision making and the anterior cingulate cortex. Nature neuroscience, 2006. 9(7): p. 940.
- Isaacson, J.S. and M. Scanziani, How inhibition shapes cortical activity. Neuron, 2011. 72(2): p. 231-243.
- Letzkus, J.J., et al., A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature, 2011. 480(7377): p. 331.
- Behrens, T.E., et al., Learning the value of information in an uncertain world. Nature neuroscience, 2007. 10(9): p. 1214.
- Croxson, P.L., et al., Effort-based cost–benefit valuation and the human brain. Journal of Neuroscience, 2009. 29(14): p. 4531-4541.
- Shenhav, A., J.D. Cohen, and M.M. Botvinick, Dorsal anterior cingulate cortex and the value of control. Nature neuroscience, 2016. 19(10): p. 1286-1291.
- Shenhav, A., M.M. Botvinick, and J.D. Cohen, The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron, 2013. 79(2): p. 217-240.
- Etzel, J.A., et al., Reward motivation enhances task coding in frontoparietal cortex. Cerebral Cortex, 2015. 26(4): p. 1647-1659.
- Braver, T.S., et al., Mechanisms of motivation–cognition interaction: challenges and opportunities. Cognitive, Affective, & Behavioral Neuroscience, 2014. 14(2): p. 443-472.
Figures