1151
Early MRS biomarkers accurately predict neurodevelopment after neonatal encephalopathy in a multicentre setting1Centre for Perinatal Neuroscience, Imperial College London, London, United Kingdom, 2Imperial College Healthcare NHS Trust, London, United Kingdom, 3Medical Physics and Bioengineering, University College London Hospitals NHS Trust, London, United Kingdom, 4Medway NHS Foundation Trust, Kent, United Kingdom, 5Department of Neonatology, Children's Hospital of Michigan, Detroit, MI, United States
Synopsis
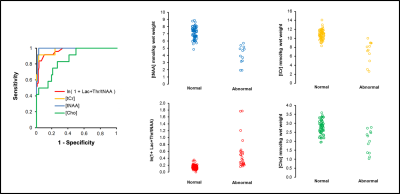
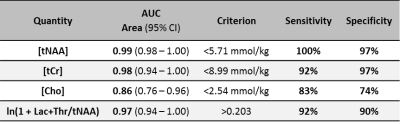
In neuroprotection trials for neonatal encephalopathy, the typical clinical outcome measures can only be measured reliably after a period of years. In a multicentre study covering eight sites and recruiting 224 infants, we demonstrate that MRS measures made within two weeks of birth provide quantitative and objective tools for predicting neurodevelopmental abnormalities usually only observed years after the initial injury. In particular, the thalamic concentration of tNAA (N-acetyl aspartate + N-acetyl aspartyl glutamate) has an area under the receiver operating characteristic curve of 0.99 (95%CI 0.98–1.00, n=82). Such tools could greatly speed up the next generation of clinical trials.
Introduction
In drug trials for neonatal encephalopathy, the clinical manifestation of therapeutic effects can only usually be demonstrated through an assessment made several years after an intervention. At this point many confounding environmental factors dilute treatment effects. With early prognostic biomarkers, there is the potential to measure brain injury and treatment effects both acutely and objectively, resulting in shorter, better powered neuroprotection trials.
In this large multicentre prospective study we examined the prognostic accuracy of early MR spectroscopy (obtained <2 weeks after birth) in predicting adverse neurodevelopment assessed by clinical examination at 18-24 months.
Methods: Study Population
We recruited consecutive term and near-term babies with neonatal encephalopathy who had therapeutic hypothermia from eight sites across the UK and USA over 3½ years1. These babies underwent one of two 3T MRS protocols (both examining a 15x15x15mm3 thalamic voxel), and had a neurodevelopmental outcome assessment (Bayley-III) at 18-24 months. Protocol A was a single acquisition at a long TE to quantify the ratio of Thr+Lac/tNAA (Thr=threonine, Lac=lactate, tNAA=NAA+NAAG=N-acetyl aspartate + N-acetyl aspartyl glutamate)2. Protocol B was a series of acquisitions allowing the quantification of the absolute metabolite concentrations, using an internal water reference.
Methods: MRS Protocols
Protocol A:
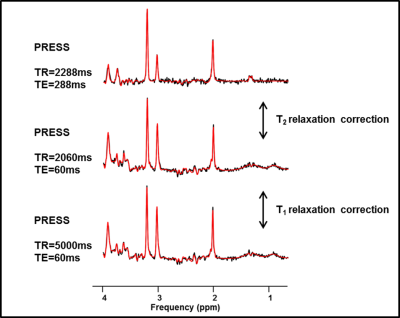
- PRESS: TR=2288ms, TE=288ms, 128 averages (comprising 16 subspectra of 8 averages each)
Protocol B:
Water suppressed:
- PRESS: TR=2288ms, TE=288ms, 128 averages (16 subspectra) - centred on NAA
- PRESS: TR=2060ms, TE=60ms, 64 averages (8 subspectra) - centred on NAA
- PRESS: TR=5000ms, TE=60ms, 64 averages (8 subspectra) - centred on NAA
Water unsuppressed:
- PRESS: TR=10000ms+TE, TE=60,124,205,316,495,1000ms - centred on H2O
Typical in vivo spectra acquired using Protocol B are shown in Figure 1.
Methods: Quality Assurance
Scanners included various models from three different vendors: Philips, Siemens and GE. The inter-site variation in MRS measurements was quantified across all scanners using a spherical phantom containing a buffered solution of 10mM NAA and 10mM Lac. In vivo quality assurance included the visual assessment of motion from imaging before and after MRS; visual assessment of voxel placement accuracy; assessment of protocol adherence; manual rejection of motion corrupted subspectra; automated spectral corrections for both frequency and phase; and the rejection of spectra with linewidths outside the normal distribution of the full dataset3.Methods: Fitting
All water suppressed spectra were analysed using LCModel (v6.3-1J), with basis sets simulated according to the TE1,TE2 combination employed by each vendor4,5. The methyl peaks of NAA, NAAG, choline (Cho), phosphocreatine (PCr) and creatine (Cr) were separated from other groups in the basis spectra to allow quantification of individual relaxation rates. NAA+NAAG were combined (tNAA), and PCr+Cr were combined (tCr) due to strong covariance during fitting.
Metabolite T1s and T2s were calculated from the fits to the spectra in Protocol B, yielding relaxation corrected signals. Water unsuppressed signals were quantified using a five component HLSVD fit6. The parenchymal water signal was quantified by biexponential fit of the water unsuppressed series7, with a long T2 component (fixed at 500ms) accounting for mobile water. Lac+Thr/tNAA results were transformed by $$$\ln{(1+x)}$$$ to reduce inherent skewness.
Results
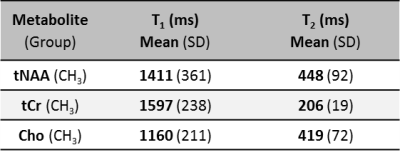
Of the 224 infants recruited, 159 had both long TE MRS and Bayley-III data available for analysis. Of these, 82 also had metabolite concentrations. The prognostic accuracy of each of Lac+Thr/tNAA, [tNAA], [tCr] and [Cho] is outlined in Table 1, with the receiver operating characteristic curves and associated scatter plots shown in Figure 2. Calculated metabolite T1s and T2s are displayed in Table 2.
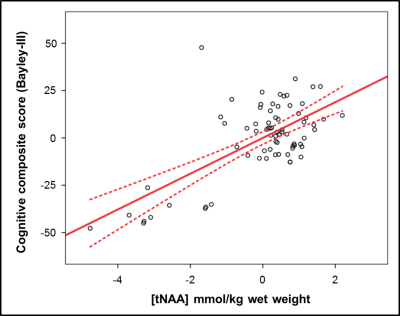
Controlling for gestational age at birth, postnatal age at scan and postnatal age at Bayley-III evaluation, [tNAA] displayed a linear relationship with cognitive composite score (Figure 3), with a 1 mmol/kg higher [tNAA] equating to 9 more points.
Discussion
The thalamic concentration of tNAA effectively predicts adverse long term neurodevelopment, and this alone accounts for 40% of the variance in cognitive performance at 18-24 months. In this study, a wide distribution of metabolite and water relaxation times were observed. The acquisitions in protocol B are designed to facilitate corrections for relaxation effects, and this is essential for accurate quantification of metabolite concentrations. Lac+Thr/tNAA, measured using a single acquisition, also predicts normal and adverse neurodevelopment. However it does so less effectively, and its skewed distribution is problematic if seeking to investigate subtle effects.Conclusion
MRS, and particularly [tNAA], provides accurate prognostic information soon after birth in neonatal encephalopathy. This is unaffected by the environmental factors which dilute the power of trials based on clinical outcome assessments made years later. The effective use of early MRS biomarkers in clinical trials could greatly reduce the time taken to translate novel putative therapies into clinical practice.Acknowledgements
Published on behalf of the MARBLE consortium. MARBLE was funded by the NIHR and the NIHR Imperial Biomedical Research Centre. PJL is supported by an NIHR Healthcare Science Doctoral Fellowship (HCS DRF-2014-05-013).
References
- Lally PJ, Pauliah S, Montaldo P et al. Magnetic Resonance Biomarkers in Neonatal Encephalopathy (MARBLE): a prospective multicountry study. BMJ Open 2015;5:e008912
- Thayyil S, Chandrasekaran M, Taylor A et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics 2010 Feb;125(2):e382-95
- Kreis R. Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR Biomed. 2004;17:361–381
- Provencher SW. Automatic quantitation of localized in vivo1H spectra with LCModel. NMR Biomed. 2001;14:260–264.
- Soher BJ, Semanchuk P, Todd D, et al. VeSPA:
Integrated applications for RF pulse design, spectral simulation and MRS data
analysis. Proc. ISMRM19 (2011):1410
- Pijanppel WWF, van den Boogart A, de Beer R, et
al. SVD-based quantification of magnetic resonance signals. J. Magn Reson.
1992;97(1):122-134
- Ernst T, Kreis R, Ross BD. Absolute Quantitation of Water and Metabolites in the Human Brain. I. Compartments and Water. J. Magn. Reson. B 1993;102(1):1–8
Figures