1149
Carnitine supplementation improves skeletal muscle acetylcarnitine formation and metabolic flexibility1Department of Radiology & Nuclear Medicine, Maastricht University Medical Center +, Maastricht, Netherlands, 2Department of Human Biology & Human Movement Sciences, Maastricht University Medical Center +, Maastricht, Netherlands, 3Department of Internal Medicine, Division of Endocrinology, Maastricht University Medical Center +, Maastricht, Netherlands
Synopsis
The formation of acetylcarnitine may serve as a mitochondrial rescue mechanism to prevent the development of metabolic inflexibility and type 2 diabetes. We here used a novel magnetic resonance spectroscopy protocol, using long echo times, to determine acetylcarnitine concentrations in skeletal muscle in vivo. Carnitine supplementation enhanced the increase in acetylcarnitine concentration in resting muscle over the day as well as the capacity to form acetylcarnitine with exercise. Furthermore, carnitine supplementation completely restored metabolic flexibility suggesting that carnitine supplementation may be an interesting aid in improving disturbed metabolism in subjects prone to develop type 2 diabetes mellitus.
Introduction
Type 2 diabetes is characterized by decreased metabolic flexibility and concomitant disturbances in glucose homeostasis. Metabolic flexibility is defined as the capacity to switch from predominantly fat oxidation in the fasted state to carbohydrate oxidation in the insulin-stimulated state. Recent evidence indicates that low carnitine availability may play an important role 1 When substrate availability is high, accumulation of intra-mitochondrial acetyl-CoA is known to blunt glycolysis, and thus leads to reduced glucose oxidation. However, acetyl-CoA can also be metabolized to acetylcarnitine, which can leave the mitochondria. Hence, impaired formation of acetylcarnitine formation may underlie compromised metabolic flexibility and therefore impair glucose tolerance (IGT). Recently, we have set up a novel, proton magnetic resonance spectroscopy (1H-MRS) protocol, using long echo times, to determine acetylcarnitine concentrations in skeletal muscle in 2 Using this approach, we here aimed to investigate in humans if carnitine supplementation leads to increased acetylcarnitine formation and improves metabolic flexibility in individuals with impaired glucose tolerance.Methods
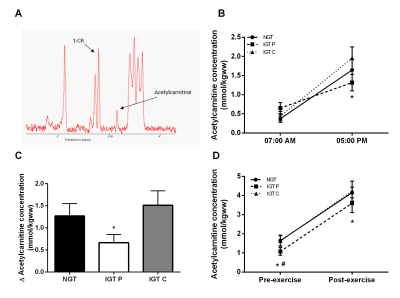
Eleven IGT volunteers followed a 36-day placebo- and L-carnitine treatment (2 g/day) in a randomized, placebo-controlled, double blind crossover design. Skeletal muscle acetylcarnitine concentrations were measured in vivo using long echo time (TE=500ms) 1H-MRS in the vastus lateralis muscle in resting state (7:00 AM and 5:00 PM) and directly after a 30-minute cycling exercise performed at 70% of the volunteers’ predetermined maximal output (Wmax). The cycling exercise was performed in the evening (5:00 PM) after five hours of fasting. All MR experiments were performed on a 3T clinical MR scanner (Achieva 3T-X, Philips Healthcare, Best, The Netherlands). Spectra were acquired with TR 6000ms, spectral bandwith 2kHz, number of acquired data points 2048, number of averages (NSA) 20 and voxel size 40x20x60mm. The creatine peak was used as internal reference for the calculation of acetylcarnitine concentrations, assuming a creatine concentration of 30mmol/kgww. T2 corrections were performed for creatine (T2=166ms) and acetylcarnitine (T2=262ms) and a correction for the dipolar coupling of creatine was applied (30% of the signal intensity). A hyperinsulinemic-euglycemic clamp (40 mU/m2/min), combined with indirect calorimetry (ventilated hood) was performed to determine metabolic flexibility. Twelve matched normal glucose tolerant (NGT) volunteers were included without any intervention as control group.Results
Skeletal muscle acetylcarnitine concentrations in the morning (7:00 AM) were not different between NGT and IGT (figure 1B) and carnitine supplementation did not affect this (figure 1B). Acetylcarnitine levels measured at 5:00 PM tended to be lower in IGT participants on placebo compared to NGT controls (1.08±0.20, 1.64±0.28 mmol/kgww for IGT and NGT respectively, p=0.06, figure 1B) and in fact, carnitine supplementation restored acetylcarnitine levels towards values observed in NGT (1.62±0.27 mmol/kgww, figure 1B). Interestingly, skeletal muscle acetylcarnitine levels were higher when measured at 5:00 PM when compared to 7:00 AM in NGT (p<0.05). This increase in skeletal muscle acetylcarnitine levels during the day (assessed in a subset of 8 participants) was markedly blunted in the IGT group (delta acetylcarnitine concentration: 0.67± 0.18 vs 1.51 ± 0.33 µmol/kgww in IGT compared to NGT, p<0.05, figure 1C), but completely restored upon carnitine supplementation (1.57 ± 0.33 mmol/kgww). Acetylcarnitine levels in skeletal muscle increased in all three groups with exercise (p<0.05), but post-exercise acetylcarnitine concentrations were markedly higher after carnitine supplementation compared to placebo (4.23±0.53 vs 3.60±0.49 mmol/kgww for carnitine and placebo respectively, p<0.05) and reached NGT-levels (4.15±0.28 mmol/kgww, figure 1D). Metabolic flexibility was lower in IGT compared to NGT (0.07±0.01 and 0.10±0.01 AU respectively, p<0.05), but was completely restored upon carnitine supplementation (0.10±0.1 AU, p<0.05).Discussion
We here show that carnitine supplementation enhanced the increase in acetylcarnitine concentration in resting muscle over the day as well as the capacity to form acetylcarnitine with exercise. Furthermore, carnitine supplementation completely restored metabolic flexibility in impaired glucose tolerant volunteers. These changes in acetylcarnitine formation may be underlying the beneficial effects on metabolic flexibility.Conclusion
Carnitine supplementation may be an interesting aid in improving disturbed metabolism in subjects prone to develop type 2 diabetes mellitus.Acknowledgements
No acknowledgement found.References
1. Muoio DM, et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012;15(5):764–777.
2. Lindeboom L, et al. Long-echo time MR spectroscopy for skeletal muscle acetylcarnitine detection. J Clin Invest. 2014;124(11):4915-4925.
Figures