1137
Imaging Brain Clearance in the Mouse Brain: Aquaporin-4 Modulates Glymphatic Inflow1Centre for Advanced Biomedical Imaging, University College London, London, United Kingdom
Synopsis
The ‘glymphatic’ system, which has recently been identified using MRI, is a brain-wide pathway for removal of waste solutes. This system is implicated in Alzheimer’s disease (AD), due to discovery that both amyloid-β and tau, accumulations that define AD, are cleared from the brain via this pathway. Using contrast-enhanced MRI, we demonstrate that glymphatic function is dependent upon aquaporin-4, a water channel located on astrocytic endfeet surrounding blood vessels in the brain. Herein, using a novel pharmacological approach, we show that aquaporin-4 represents a suitable drug target for manipulation of glymphatic function in the brain, and in treatment of AD.
Introduction
The recently discovered glymphatic clearance system describes the movement of cerebrospinal fluid (CSF) into the brain’s parenchyma, via a para-arterial influx pathway, where it convectively exchanges with interstitial fluid (ISF) and is cleared from the brain via a para-venous efflux mechanism, taking interstitial solutes with it [1]. Failure of this system has been implicated in Alzheimer’s disease (AD), due to discovery that amyloid-β and tau, brain accumulation of which neuropathologically define AD, are cleared from the brain via this system. Appropriately, we showed recently using dynamic contrast-enhanced MRI, that this system is specifically impaired in the brains of transgenic mice modelling parenchymal protein accumulation [2], and hypothesise that this pathway may contribute to pathogenic accumulation of proteins in the AD brain. However, recent controversies have arisen in the field as to the use of such imaging methods to study convective glymphatic exchange of CSF with ISF, in that researchers have been unable to reproduce the initial results of experiments used to define the glymphatic pathway. Namely, that genetic deletion of the water channel aquaporin-4 (AQP4), which resides on astrocytic endfeet that surround blood vessels in the brain, perturbs glymphatic function, and clearance of amyloid-β from the brain by ~70% [3]. Here, rather than utilising a genetic knock-out of this AQP4 channel, which is thought vital for appropriate function of the glymphatic clearance pathway, we demonstrate that a novel pharmacological AQP4 inhibitor (TGN-020) is capable of supressing glymphatic function, assessed through contrast-enhanced MRI. Moreover, we demonstrate that a markedly similar level of impairment is observed in clearance of tau protein from the brain after treatment of mice with TGN-020, suggesting that contrast-enhanced MRI measures of glymphatic function are a viable measure of parenchymal clearance of waste protein species. Furthermore, our data demonstrate the druggability of this AQP4 channel in the brain and in AD, and provide evidence that pharmacological manipulation of AQP4 alters the function of the glymphatic clearance pathway in its removal of tau from the brain.Methods
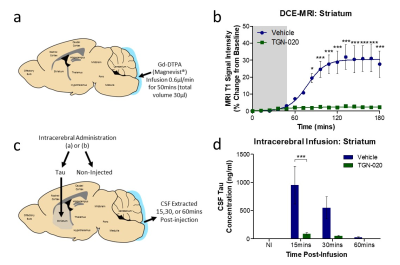
Glymphatic inflow in the brains of C57BL/6 mice was quantified using dynamic contrast-enhanced MRI, via intrathecal administration of Gd-DTPA into the caudal most CSF filled region of the brain, the cisterna magna. Gd-DTPA was infused concurrent with acquisition of whole brain T1-weighted MR images, for a total time of 180mins. Mice were treated with either AQP4 inhibitor (TGN-020, 250mg/kg, i.p.) or vehicle (20% Captisol) 15mins prior to infusion. A separate cohort of mice was intracerebrally infused with tau-containing brain homogenate 15mins after drug/vehicle treatment, and CSF collected to assess extent of tau clearance from brain parenchyma to CSF compartments during pharmacological AQP4 blockade.Results
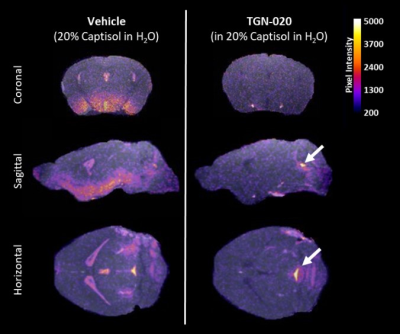
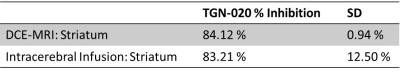
Glymphatic inflow of Gd-DTPA into the parenchyma was significantly supressed after TGN-020 treatment. Contrast was observed in caudal aspects of the ventricular system throughout infusion of Gd-DTPA into the cisterna magna (fig.1, white arrows) yet its parenchymal penetration was significantly ablated in the brain compared to vehicle treated animals. Quantification of contrast-enhanced signal intensity in the striatum after Gd-DTPA infusion (fig.2a,b), revealed almost complete lack of signal enhancement in this brain region after TGN-020 treatment (fig 2b), with 84.12±0.93% inhibition in signal enhancement compared to vehicle treated animals. Similarly, intracerebral infusion of tau-containing brain homogenate into the striatum (fig.2c), revealed that TGN-020 treatment significantly reduced tau clearance from this region of the brain’s parenchyma into CSF compartments, as early as 15mins after parenchymal infusion (90.1±48.3mg/ml CSF tau in TGN-020 treated mice, compared to 953.0±754.5mg/ml in vehicle treated mice, p<0.001), an effect which was also observed, 30, and 60mins post-infusion (fig.2d). Comparison of the level of inhibition exerted by TGN-020 on parenchymal inflow of Gd-DTPA, and clearance of tau from the brain (table1), is suggestive that contrast-enhanced MRI measures of glymphatic function are a viable measure of parenchymal clearance of waste protein species, and that this this agent is capable of inhibiting both arterial and venous associated AQP4 channels equally.Discussion and Conclusions
The data presented here demonstrates that pharmacological blockade of AQP4 via treatment with a potent novel inhibitor, TGN-020, suppresses both glymphatic inflow of MR contrast agent, Gd-DTPA, into the brain, and the clearance of tau from the brain. Additionally, the finding that both inflow and clearance from the brain are equally as affected by TGN-020 pre-treatment, is suggestive that this agent is capable of inhibiting both arterial and venous associated AQP4 channels equally, and that contrast-enhanced MRI is an appropriate surrogate measure of glymphatic clearance of waste proteins from the brain. Moreover, these findings suggest that pharmacological manipulation of AQP4 alters the function of the glymphatic clearance pathway in its removal of tau from the brain, positioning AQP4 as a novel drug target for the treatment of AD.Acknowledgements
This work is funded by research grants from the UK's Engineering and Physical Sciences Research Council (EPSRC) (EP/N034864/1) and Eli Lilly and Company.References
[1] Iliff, J.J. et al. (2012), SciTransMed, 4(147):p.147.
[2] Harrison, I.F. et al., (2016), ISMRM Conference Proceedings, Abstract #4442.
[3] Smith, A.J. et al., (2017), eLife, 6:e27679.
Figures