1133
The travelling pulses: multicenter evaluation of universal pulses at 7T1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2NeuroSpin, CEA, Saclay, France, 3Siemens Healthcare, Saint-Denis, France, 4German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany
Synopsis
It has been shown recently that parallel transmission universal pulses (UPs), optimized offline on a training field-maps database, can be used to robustly mitigate B1+ inhomogeneity on other subjects, thus holding great potential for spreading their utility by sparing the user the time-consuming individualized calibration. For these UPs to be used widely, however their performance needs to be immune to inter-site differences. In this study, we examined the robustness of the UPs against inter-site variability. Our results so far obtained at two sites show that the UPs are quite robust in producing uniform contrast across the brain despite these differences.
Introduction
Although parallel transmission (pTx) has great utility for mitigating radiofrequency field inhomogeneity at ultra-high field, its use in routine scans has remained marginal due to a cumbersome calibration procedure that requires subject-specific field mapping and pulse design. Recently, a novel method aiming at providing calibration-free solutions by designing universal pulses (UPs) was proposed to skip the entire calibration procedure while still being able to counteract the RF inhomogeneity problem in an effective and safe way1,2. The wide use of this new method however requires the pulses to be immune to inter-site variability, which is known to exist due to slight variations in tune-up across sites3. The purpose of this work was to examine how robust the non-selective UPs are against inter-site variability.Methods
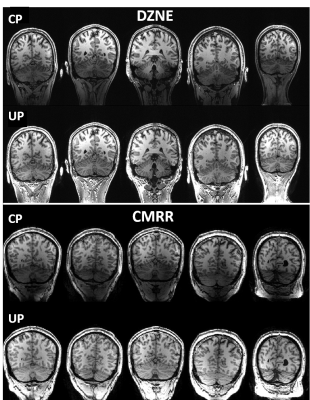
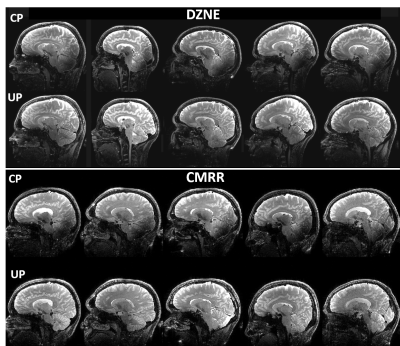
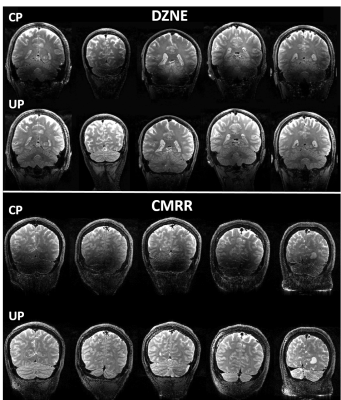
Universal pulse design aims at calculating RF solutions that are robust against inter-subject variability. Universal pulses were used at 7T for brain imaging at two sites: 1) the German Center for Neurodegenerative Diseases (DZNE, Bonn, Germany) and 2) the Center for Magnetic Resonance Research (CMRR) at the University of Minnesota. At both sites, brain images were acquired on a 7T Magnetom scanner (Siemens Healthcare, Erlangen, Germany) equipped with the SC72 gradient and using the commercially available Nova 8Tx-32Rx head coil (Nova Medical, Wilmington, MA, USA). At DZNE, a database of 10 adult subjects (5 men, 5 women, age=40±15 years) were first scanned to return their respective transmit B1 and B0 field maps. These maps were used to design non-selective UPs for T1w MPRAGE and T2w SPACE sequences. In particular, they were used to design UPs for inversion (MPRAGE), excitation (MPRAGE, SPACE) and refocusing (SPACE) pulses. These UPs were loaded by the modified pTx-enabled MPRAGE and SPACE sequences for data acquisition. All UPs were based on the kT-points parametrization and were designed with simultaneous optimization of the k-space trajectory4 under explicit hardware and SAR constraints based on Virtual Observation Points (VOPs). The cost-function of the minimization algorithm was the flip angle (FA) Normalized Root Mean Square Error averaged over the 10 database subjects. The relevant imaging parameters for the MPRAGE protocol were: 1 mm isotropic resolution, TI=1.1s, TR=2.6s, ETL=160, iPAT=2, FA=5°, TA=4:28. For the SPACE protocol, relevant imaging parameters were: 0.8 mm isotropic resolution, TR=3s, ETL=117, iPAT=2, Partial Fourier 6/8, TA=7:48 with variable FA train5. After offline pulse design, the calculated UPs were blindly applied at DZNE to acquire brain images from new subjects (10 subjects for MPRAGE2 and 5 subjects for SPACE3), without any subject-specific pTx calibration. The exact same protocols including the same UPs and same setups were then utilized at the CMRR to acquire brain images on 5 additional adult subjects (4 men, 1 woman, 18 < age < 62) to examine the robustness of the UPs against inter-site variability. For comparison, brain images were also acquired from the same subjects at each site using the Circularly-Polarized (CP) mode, with the average FA over a central axial slice reaching the nominal FA.Results
At both sites, the use of UPs to acquire MPRAGE images led to largely improved tissue contrast across the entire brain, especially in such lower brain regions as temporal lobes and cerebellum (Figs. 1 and 2); this improvement was observed in all of the five subjects shown. More strikingly, the use of UPs to acquire SPACE images effectively restored signals in the lower brain regions including temporal lobes, occipital lobes and cerebellum, leading to substantially improved tissue contrast across the entire brain; again this improvement was seen in all of the 5 subjects shown. The peak 10g SAR values of the UPs, estimated with the VOPs, were 5.6 W/kg for MPRAGE and 7.9 W/kg for SPACE acquisitions.Discussion and conclusion
Here we have shown that the nonselective UPs designed using a database of pTx field maps acquired at one site (DZNE) can be blindly applied not only at the same site but also at another site (CMRR) to collect high quality, high resolution whole brain structural T1w and T2w images at 7T. Despite the existing inter-site variability, our results show that the use of UPs can robustly and substantially improve the tissue contrast across the entire brain at both sites, without requiring the cumbersome pTx calibration procedure. To further validate the universality of these pulses, we welcome more sites to join us in testing these UPs so that the travelling pulses can continue to travel along their journey toward being widely accepted as a calibration-free, B1+ mitigation solution that can benefit a large array of neuroimaging applications at ultrahigh field.Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Program, Proof of Concept Grant Agreement n. 700812 and from NIH grants including U01 EB025144 and P41 EB015894.References
[1] V. Gras, M. Boland, A. Vignaud, G. Ferrand, A. Amadon, F. Mauconduit, D. Le Bihan,T. Stöcker, N. Boulant. Homogeneous non-selective and slice-selective parallel-transmit excitations at 7 Tesla with universal pulses: A validation study on two commercial RF coils. PLoS ONE 12(8): e0183562.
[2] V. Gras, F. Mauconduit, A. Vignaud, A. Amadon, D. Le Bihan, T. Stöcker, N. Boulant. Design of universal parallel-kT-point pulses and application to three-dimensional T2-weighted imaging at 7T. DOI:10.1002/mrm.27001.
[3] M. Voelker, O. Kraff, D. Brenner, A. Wollrab, O. Weinberger, MC. Berger, S. Robinson, W. Bogner, C. Wiggins, R. Trampel, T. Stöcker, T. Niendorf, HH. Quick, D.G. Norris, M.E.Ladd, O. Speck. The traveling heads: multicenter brain imaging at 7 Tesla. MAGMA. 2016 29:399-415.
[4] V. Gras, M. Luong, A. Amadon, N. Boulant. Joint design of kT-points trajectories and RF pulses under explicit SAR and power constraints in the large flip angle regime. JMR 261 (2015) 181-189.
[5] JP. Mugler. Optimized three-dimensional fast-spin-echo MRI. J. Magn. Reson. Imaging 2014;39:745–767. doi: 10.1002/jmri.24542.
Figures