1118
Quasiperiodic Patterns in BOLD fMRI Reflect Neuromodulatory InputAnzar Abbas1, Maysam Nezafati2, Isak Thomas2, and Shella Keilholz1,2
1Neuroscience, Emory University, Atlanta, GA, United States, 2Biomedical Engineering, Emory University and Georgia Institute of Technology, Atlanta, GA, United States
Synopsis
The mechanisms behind spontaneous activations in the BOLD signal are not understood. Quasiperiodic patterns are reliably recurring spatiotemporal events that involve spontaneous fluctuations of brain regions. We hypothesize that quasiperiodic patterns are regulated by neuromodulatory inputs from a subcortical driver. Pharmaceutical modulation of the locus coeruleus resulted in reduction of the strength and frequency of quasiperiodic patterns in the BOLD signal. This indicates a direct relationship between the locus coeruleus and quasiperiodic patterns. These findings suggest that spontaneous activity in the brain may be arising from controlled neuromodulatory input, allowing for a clearer understanding of the mechanisms that drive it.
Introduction
The mechanisms behind spontaneous BOLD fluctuations are not understood1. We have identified a quasiperiodic pattern (QPP) in the brain, which involves spontaneous activation of brain regions in critical networks, chiefly the default mode network (DMN) and the task positive network (TPN)2,3. QPPs were first observed in anesthetized rats2, and have since been recorded in mice4, rhesus macaques5, and humans3 using a pattern-finding algorithm. Low-frequency patterns such as QPPs are major contributors to functional connectivity6, which is disrupted during neurological disorders7. QPPs have recently been shown to occur with greater strength and frequency in awake states compared to anesthetized states in macaques5. This, in addition to the involvement of the DMN and TPN, indicates a possible relationship between QPPs and arousal. Large-scale modulation of neural activity, as seen in QPPs, is likely caused by an external driver. Noradrenergic innervation, stemming from locus coeruleus (LC) activity, has been shown to be important for arousal8. We hypothesize that LC activity regulates QPPs and that a pharmaceutical disruption of the LC will result in irregular or reduced QPP activity. If so, QPPs may provide novel ways to study large-scale modulations originating from subcortical nuclei.Methods
9 male rats were anesthetized with isoflurane and placed in a 9.4T Bruker MRI scanner, where anesthesia was maintained with a combination of isoflurane and dexmedetomidine. For each subject, one anatomical image (FLASH3D sequence; TR 70ms, TE 4ms, FOV 3.2cm, matrix size 128x128x128) and 1-n functional timeseries (GE-EPI sequence; TR 1000ms, TE 14.5ms, FOV 3.2cm, matrix size 64x64x30) were acquired. 10 days before scanning, 4 rats had been injected with 50mg/kg DSP4 to decrease noradrenergic innervation of the cortex to 10-20% of its normal value and reduce the LC basal firing rate by 50%9. 30 minutes prior to scanning, 2 separate rats had been administered with 1mg/kg i.p. Atomoxetine to increase extracellular norepinephrine levels and signaling in the cortex10. 3 rats were used as controls. All data was preprocessed through slice time correction, motion correction, registration to standard space, 0.5mm Gaussian kernel spatial smoothing, 0.01-0.25Hz bandpass temporal filtering, global signal regression, and z-scoring. A pattern-finding algorithm was applied to all functional timeseries with the parameters described in Majeed et al. (2011)3. The algorithm outputs any repeating event such as a QPP and a sliding window correlation of the event with the functional timeseries it was obtained from. The sliding window correlation vectors were compared between all scans. For each group, the sliding window correlations from all scans were concatenated and their values were plotted as a histogram in order to compare the strength and frequency of the event over time.Results
Application of a pattern-finding algorithm resulted in a reliably recurring spatiotemporal event in the control group (n=7 scans) aligning with observations from previous studies conducted in rats2,3. The sliding window correlation vector shows robust repetitions of this event over the course of the functional timeseries (Fig1a). Application of the pattern-finding algorithm in the DSP4 group (n=9) and the Atomoxetine group (n=10) did not result in a reliably recurring spatiotemporal pattern. The sliding window correlation vectors from the experimental groups did not show robust repetition in the functional timeseries (Fig1b, Fig1c). Histograms for each group reflected this difference, with wider deviations from zero seen in sliding window correlation vectors from the control group versus treatment groups (Fig2). Far from being a negative finding, a lack of pattern supports our hypothesis that LC innervation regulates QPP occurrence.Conclusions
Results from the pattern-finding algorithm in the control group align with QPPs that have been observed in anesthetized rats in previous studies2,3. Pharmaceutical modulation of LC activity through DSP4 and Atomoxetine affected these results: Administration of DSP4 led to reduced visibility of a reliably recurring event in the functional timeseries. This is expected as DSP4 is a suppressor of LC activity9. However, administration of Atomoxetine, which is a norepinephrine re-uptake inhibitor10, also resulted in reduced visibility of a reliably recurring event. This suggests that a tonic level of input from the LC may be regulating QPP occurrence. This would align with our observations as both DSP4 and Atomoxetine reduce baseline tonic firing rate of the LC. Our results show that low-frequency patterns of activity in the brain, such as QPPs, can be influenced by activity from subcortical inputs. This allows a clearer understanding of drivers of spontaneous activity in the brain.Acknowledgements
No acknowledgement found.References
- Hutchison, R. M., Womelsdorf, T., Allen, E. A., Bandettini, P. A., Calhoun, V. D., Corbetta, M., et al. (2013). Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage, 80, 360–378. http://doi.org/10.1016/j.neuroimage.2013.05.079
- Majeed, W., Magnuson, M., & Keilholz, S. D. (2009). Spatiotemporal dynamics of low frequency fluctuations in BOLD fMRI of the rat. Journal of Magnetic Resonance Imaging, 30(2), 384–393. http://doi.org/10.1002/jmri.21848
- Majeed, W., Magnuson, M., Hasenkamp, W., Schwarb, H., Schumacher, E. H., Barsalou, L., & Keilholz, S. D. (2011). Spatiotemporal dynamics of low frequency BOLD fluctuations in rats and humans. NeuroImage, 54(2), 1140–1150. http://doi.org/10.1016/j.neuroimage.2010.08.030
- Belloy, M., Naeyaert, M., Keliris, G., Abbas, A., Keilholz, S., Van Der Linden, A., Verhoye, M. Dynamic resting state fMRI in mice: detection of Quasi‐Periodic Patterns. Proceeding Int. Soc. Magn. Reson. Med. p.0961. (2017).
- Abbas, A., Langley, J., Howell, L., Keilholz, S. Quasiperiodic patterns vary in frequency between anesthetized and awake monkeys. Resting State and Brain Connectivity 2016. http://www.restingstate.com/2016/abstracts/
- Thompson, G. J., Pan, W.-J., Billings, J. C. W., Grooms, J. K., Shakil, S., Jaeger, D., & Keilholz, S. D. (2014). Phase-amplitude coupling and infraslow (<1 Hz) frequencies in the rat brain: relationship to resting state fMRI. Frontiers in Integrative Neuroscience, 8, 41. http://doi.org/10.3389/fnint.2014.00041
- Mohan, A., Roberto, A., Mohan, A., Lorenzo, A., Jones, K., Carney, M., et al. (2016). The Significance of the Default Mode Network (DMN) in Neurological and Neuropsychiatric Disorders: A Review. Yale Journal of Biology and Medicine, 1–9.
- Aston-Jones G, Waterhouse B. Locus Coeruleus: From Global Projection System to Adaptive Regulation of Behavior. Brain Res. 2016:1-4. doi:10.1016/j.brainres.2016.03.001.
- Ross, S. B., & Stenfors, C. (2014). DSP4, a Selective Neurotoxin for the Locus Coeruleus Noradrenergic System. A Review of Its Mode of Action. Neurotoxicity Research, 27(1), 15–30. http://doi.org/10.1007/s12640-014-9482-z
- Bari, A., & Aston-Jones, G. (2013). Atomoxetine modulates spontaneous and sensory-evoked discharge of locus coeruleus noradrenergic neurons. Neuropharmacology, 64(c), 53–64. http://doi.org/10.1016/j.neuropharm.2012.07.020
Figures
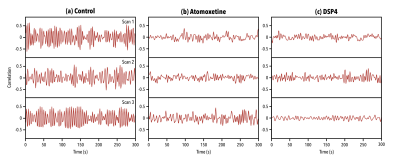
Figure 1. Examples of sliding window correlation (SWC) vectors of repeating events with the functional timeseries they were obtained from. (a) SWC vectors of QPPs acquired from the control group in three separate scans. (b) SWC vectors of events acquired from the Atomoxetine group in three separate scans. (c) SWC vectors of events acquired from the DSP4 group in three separate scans. SWC vectors in (a) show a robust repetition of an event, while (b) and (c) show reduction in strength and frequency of the observed event.
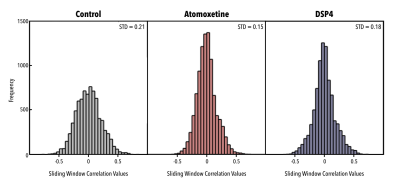
Figure 2. Histograms of concatenated sliding window correlation (SWC) vectors from each group. Shorter, wider histograms suggest a greater standard deviation from zero, indicating a stronger and more frequently repeating event. Slimmer, taller histograms suggest a smaller standard deviation from zero, indicating a weaker, less frequently repeating event. Events outputted from the pattern-finding algorithm occur with greater strength and frequency in the control group compared to both experimental groups.