1116
Distinct structure-function relationships at different hierarchical levels of structural connectivity in the rat brain.1Biomedical MR Imaging and Spectroscopy group, Center for Image Sciences, University Medical Center Utrecht and Utrecht University, Utrecht, Netherlands, 2Department of Translational Neuroscience, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht, Netherlands, 3Department of Anatomy, University of Rostock, Rostock, Germany, 4Department of Pediatric Neurology, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
The relationship between functional and structural connectivity strength in the brain remains uncertain. We compared high-field resting-state fMRI, diffusion-based tractography and neuronal tracer data to robustly characterize the rat connectome. Our study revealed that strong structural connectivity is not required for strong functional connectivity. We found distinct structure-function relationships at different hierarchical levels in the rat brain: functional connectivity strength correlated moderately with diffusion-based structural connectivity strength, but did not significantly correlate with neuronal tracer-based structural connectivity strength. Hereby we demonstrate the importance of examining or appraising connectivity at different hierarchical levels for reliable assessment of neural network organization.
Introduction
Connectivity between brain regions can be determined with several techniques. Structural connectivity can be measured at the macro-scale with diffusion-based tractography and at the meso-scale with neuronal tracers. Functional connectivity can be measured at the macro-scale with resting-state fMRI. Combining techniques at different hierarchical levels may help to better characterize the complex organization of the brain, which is crucial to understand brain connectivity in health and disease. Structural and functional connectivity strength partly correlate1,2, suggesting that structural connectivity provides the hardware for functional connectivity to emerge. However, so far most studies included structural connectivity measured at either the meso- or macro-scale, and thereby did not capture all aspects of structural connectivity.Therefore, the aim of our study was to robustly characterize the rat connectome by integrating all three connectivity measures based on high-field resting-state fMRI, diffusion-based tractography and neuronal tracer data, respectively. This allows the investigation of functional connectivity within the strongest structural connections at the meso- and macro-scale, with general exclusion of false positives and negatives often present in diffusion-based tractography data3.
Methods
Resting-state functional connectivity was determined in two groups of male rats: young adult Sprague Dawley rats (n=18, mean age 14 weeks: Group I) and adult Wistar rats (n=12, mean age 32 weeks: Group II). Diffusion-based structural connectivity was determined in ten post-mortem brains from adult male Wistar rats. MRI measurements were performed on a 9.4T horizontal bore Varian MR system. Resting-state fMRI was performed under 1.5% isoflurane anesthesia using a T2*-weighted single-shot 3D gradient EPI sequence (TR/TE = 26.1/15 ms; 13° flip angle, 54×54×28 matrix; 600µm isotropic voxels, 800 volumes; 730.8 ms per volume). Post-mortem diffusion MRI was performed with a 3D 8-shot EPI sequence (TR/TE = 500/32.4 ms; field-of-view = 19.2x16x33 mm3; 150µm isotropic voxels; 5 b0 images; diffusion-weighting in 60 non-collinear directions with b = 3842 s/mm2; four averages).Preprocessing of resting-state fMRI images included motion correction, regression of motion correction parameters and band-pass filtering (0.01<f<0.1 Hz). Functional connectivity strength was measured as Fisher's z-transformed correlation coefficient. Diffusion-based structural connectivity strength was determined with single shell constrained spherical deconvolution tractography and expressed as the streamline count corrected for underlying white matter densities using SIFT4. Diffusion-weighted MRI and resting-state fMRI scans were non-linearly registered using FSL5 to a 3D model of the Paxinos and Watson rat brain atlas6. 84 cortical and 8 subcortical regions covering the majority of the rat brain were projected onto the individual scans. Neuronal tracer-based structural connectivity strength was extracted from the NeuroVIISAS database (generated from 7800 published tract-tracing studies) for the same regions, and expressed as values between 0 (no information) and 4 (very strong connection)7.
Results
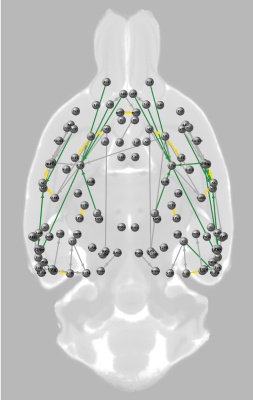
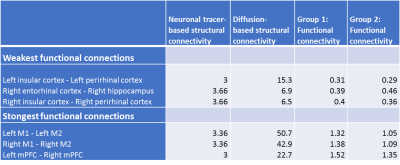
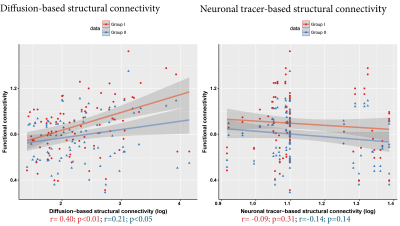
1921 connections between the selected regions-of-interest had a neuronal tracer-based structural connectivity strength > 0, of which 1470 connections also had a diffusion-based structural connectivity strength > 0 (76.5%). For subsequent analyses, we only included connections that belonged to the 25% strongest diffusion-based and 25% strongest neuronal tracer-based structural connections, visualized as the structural core of the rat connectome at the meso- and macro-scale in Figure 1. Within this structural core, functional connectivity strength was 0.31<z<1.52 for Group I and 0.29<z<1.35 for Group II.For the included structural connections, lowest functional connectivity was found for intrahemispheric connections between perirhinal, entorhinal and insular cortex and the hippocampus, for both Groups I and II (Figure 2). These regions demonstrated higher functional connectivity within less strongly structural connections. Highest functional connectivity was found for intrahemispheric connections between primary and secondary motor cortices and the connection between the left and right medial prefrontal cortex. Functional connectivity strength correlated moderately with diffusion-based structural connectivity strength (Group I: r=0.40; Group II: r=0.21; p<0.05), but not with neuronal tracer-based structural connectivity strength (Group I: r=-0.09; Group II: r=-0.14) (Figure 3).
Discussion
Within the strongest structural connections at both the meso- and macro-scale, we found a large variation of functional connectivity. This suggests that strong direct structural connections on different hierarchical levels are not required for strong functional connections. Within these strongest structural connections, functional connectivity between primary areas like sensorimotor areas is high, whereas functional connectivity between higher order cognitive areas is low. Our correlations between connectivity measures at macro- and meso-scale agree with another study8, showing that functional connectivity strength correlates moderately with macro-scale but not clearly with meso-scale structural connectivity strength. Substantially different techniques to measure connectivity could be responsible for the missing correlation between functional and meso-scale neuronal tracer-based structural connectivity. In conclusion, our study demonstrates the importance of examining connectivity at different hierarchical levels to better understand brain organization.Acknowledgements
No acknowledgement found.References
1 Honey et al.
Predicting human resting-state functional connectivity from structural
connectivity. PNAS 2009; 106(6); 2035-2040.
2 Miranda-Dominguez et al.
Bridging the gap between the human and macaque connectome: A quantitative
comparison of global interspecies structure-function relationships and network
topology. The Journal of Neuroscience 2014; 35(16); 5552-5563.
3 Maier-Hein et al. Tractography-based
connectomes are dominated by false-positive connections. BioRxiv 2016; 1-23.
4 Smith et al. SIFT:
Spherical-deconvolution informed filtering of tractograms. NeuroImage 2013, 67,
298-312.
5 Jenkinson et al. FSL.
NeuroImage 2012; 62; 782-790. www.fmrib.ox.ac.uk/fsl
6 Paxinos & Watson. The rat brain. 2005.
7 Schmitt & Eipert. NeuroVIISAS: Approaching multiscale
simulation of the rat connectome. Neuroinformatics 2012; 10; 243-267.
8 Reid et al. A
cross-modal, cross-species comparison of connectivity measures in the primate
brain. NeuroImage 2016; 125; 311-331.
Figures