1111
Neural activity pattern(s) underlying brain interhemispheric propagation: An optogenetic fMRI study1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China
Synopsis
The sensory system is topographically organized by highly interconnected excitatory thalamo-cortical and interhemispheric cortical-cortical projections. However, little is known at present regarding the existence of spatiotemporal neural activity pattern(s) that may dictate the propagation of activity between cortices at both hemispheres. Here, we employed a novel paired-pulse optogenetic fMRI stimulation paradigm to reveal a temporally-specific neural activity pattern initiated from the somatosensory thalamus that could drive activity propagation from the ipsilateral to contralateral cortex. We found that propagation from the ipsilateral to contralateral somatosensory cortex was facilitated when paired optogenetic stimulation pulses were spaced at 100ms, but not 50ms.
Purpose
One hallmark of long-range excitatory projections in the brain is the presence of the corpus callosum, a massive white-matter structure that interconnects the two cortical hemispheres1. Interhemispheric callosal/cortico-cortical projections predominantly interconnect sensory cortices2. Recently, it has been shown that neural activity inputs from the thalamus are critical for the development of inter- and intracortical laminar organization3. These studies suggest the importance of structural and functional integrity in the thalamo-cortical and interhemispheric cortico-cortical projections as prerequisites for normal sensory processing. However, little is known at present regarding the spatiotemporal neural activity pattern(s) initiated from the thalamus that may dictate the propagation of activity between the sensory cortices at both hemispheres. In this study, we employed a novel paired-pulse optogenetic fMRI stimulation paradigm to activate ventral posteromedial thalamus (VPM) excitatory thalamocortical neurons. We aim to reveal the temporally-specific neural activity pattern that could drive activity propagation from the ipsilateral to contralateral hemisphere using the well-defined topographically-organized somatosensory thalamo-cortical network as our model.Methods
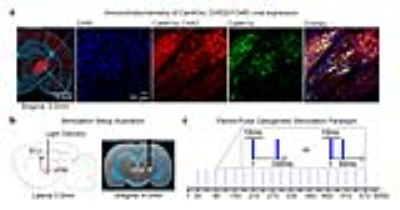
Animal preparation and optogenetic stimulation: 3μl of AAV5-CaMKIIα::ChR2(H134R)-mCherry was injected to VPM of adult rats (200-250g, male, SD strain, n=6; Figure 1a). Four weeks after injection, an opaque optical fiber cannula (d=450μm) was implanted at the injection site (Figure 1b). Blue (473nm) light was presented to animals expressing ChR2 with a paired-pulse paradigm (10ms pulse width, interstimulus interval, ISI=100ms or 50ms, 40mW/mm2; Figure 1c).
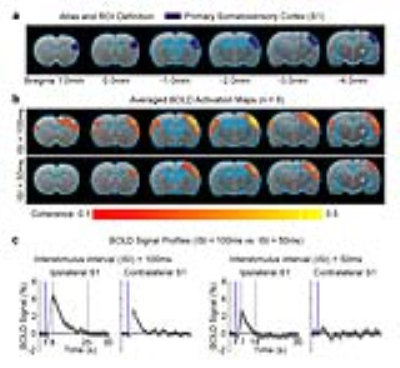
fMRI acquisition and analysis: fMRI data was acquired at 7T using GE-EPI (FOV=32×32mm2, matrix=64×64, α=56°, TE/TR=20/1000ms, 16 contiguous slices with 1mm thickness). Data were preprocessed before coherence analysis was applied to identify significant BOLD responses (p<0.001). BOLD signal profiles were extracted from anatomically defined ROI.
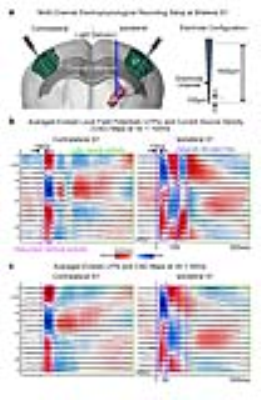
Electrophysiological recordings and analysis: Recordings at bilateral S1 were performed using linear microelectrode arrays (n=3; 16 electrode channels equally spaced at 100μm, 1.5MΩ; sampled at 30kHz). Recorded data was downsampled to 2kHz, band-pass filtered (1-300Hz) and notch filtered for 50Hz before further local field potential (LFP) and current source density (CSD) analyses were performed.
Results
Pulsed optogenetic stimulation at the VPM thalamocortical excitatory neurons activated the ipsilateral primary somatosensory cortex (S1; Figure 2). However, the BOLD response was stronger (4% vs. 2%), reached its peak later (8s vs. 7s) and prolonged (25s vs. 14s) when the ISI of the paired-pulse was 100ms in comparison to 50ms. This finding indicates differences in the underlying evoked neural activity in ipsilateral S1. Interestingly, we detected robust BOLD activation at the contralateral S1 when the ISI was 100ms, but not 50ms.
Furthermore, we examined the spatiotemporal characteristics of neural activity propagation between the bilateral S1, initiated at the VPM. We performed multi-channel depth electrophysiological recordings at the ipsilateral and contralateral S1 (Figure 3a). We measured a latency of ~13ms between the onset of the first optogenetic pulse and the first evoked LFP in L4; indicating direct propagation through thalamo-cortical projections (Figure 3b). An additional latency of ~6ms was measured before the first evoked LFP was recorded at L2/3 and L5 of the contralateral S1. These results corroborate the conduction delays across interhemispheric cortico-cortical projections4,5. We then generated CSD maps from the LFP recordings (Figure 3b, c). It was observed that long-lasting current source and sink patterns (~50ms) were successfully evoked across the layers in the bilateral S1 by the paired optogenetic pulses with ISI=100ms (Figure 3b). However, only the first optogenetic pulse evoked such a source and sink pattern with ISI=50ms (Figure 3c).
Discussion and Conclusion
In this study, we demonstrate that by probing the somatosensory thalamo-cortical network with a paired-pulse stimulation paradigm, a temporally-specific interhemispheric neural activity propagation pattern was revealed. We found that robust positive BOLD responses were weaker and shorter in ipsilateral S1, and absent in the contralateral S1 when the ISI was decreased from 100ms to 50ms. These findings indicate that the underlying neural activity that occurs at long time-scales may be absent. Multi-channel depth recordings revealed that when ISI=50ms, ipsilateral S1 did not respond to the second optogenetic pulse and bilateral S1 lacked the characteristic late neural activity (>100ms after onset of stimulation; Figure 3b, c). Interestingly, we observed long-lasting neural activity (~50ms) evoked by the first optogenetic pulse across all S1 layers. This suggests the presence of recurrent cortical activity, which has been reported to require long-time windows for integration across layers6. Such activity in S1 may impede any subsequent response to the second optogenetic pulse at ISI=50ms and disrupt propagation to the contralateral S1. In conclusion, our work offers a novel optogenetic fMRI approach to investigate neural activity propagation dynamics in the brain.Acknowledgements
This work was supported by the Hong Kong Research Grant Council (Grants C7048-16G and HKU17103015 to E.X.W.).References
- Somogyi, P., Tamas, G., Lujan, R. & Buhl, E.H. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev 26, 113-135 (1998).
- Zhou, J., Wen, Y., She, L., Sui, Y.-n., Liu, L., Richards, L.J. & Poo, M.-m. Axon position within the corpus callosum determines contralateral cortical projection. Proceedings of the National Academy of Sciences 110, E2714-E2723 (2013).
- Jabaudon, D. Fate and freedom in developing neocortical circuits. Nat Commun 8, 16042 (2017).
- Leong, A.T., Chan, R.W., Gao, P.P., Chan, Y.S., Tsia, K.K., Yung, W.H. & Wu, E.X. Long-range projections coordinate distributed brain-wide neural activity with a specific spatiotemporal profile. Proc Natl Acad Sci U S A 113, E8306-E8315 (2016).
- Debanne, D., Campanac, E., Bialowas, A., Carlier, E. & Alcaraz, G. Axon physiology. Physiol Rev 91, 555-602 (2011).
- Reinhold, K., Lien, A.D. & Scanziani, M. Distinct recurrent versus afferent dynamics in cortical visual processing. Nat Neurosci 18, 1789-1797 (2015).
- Lee, J.H., Durand, R., Gradinaru, V., Zhang, F., Goshen, I., Kim, D.S., Fenno, L.E., Ramakrishnan, C. & Deisseroth, K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788-792 (2010).
- Quairiaux, C., Megevand, P., Kiss, J.Z. & Michel, C.M. Functional development of large-scale sensorimotor cortical networks in the brain. J Neurosci 31, 9574-9584 (2011).
Figures