1110
Simultaneous fMRI and Fast-Scan Cyclic Voltammetry: Methodological Considerations and In Vivo Oxygen Measurements1Biomedical Research Imaging Center and Department of Neurology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Synopsis
fMRI is based on the dogma that neuronal activity couples to local hemodynamic changes; however, exceptions to this rule exist with little explanation why. Additional neurophysiological context, such as concurrent release of vasoactive neurotransmitters, is required to discern how other underlying factors contribute to evoked hemodynamic responses. Fast-scan cyclic voltammetry (FSCV) is a minimally-invasive technique capable of detecting neurotransmitters and tissue oxygen with high temporal and spatial resolution. Here, we design a multimodal platform to perform simultaneous fMRI and FSCV, prove its feasibility in vitro, and expand its use to characterize evoked oxygen detection in vivo.
Introduction
BOLD fMRI has been a cornerstone of dissecting brain functionality by measuring the cerebral blood oxygenation changes in response to stimuli.1 Though most changes are attributed to neuronal activity, the roles of vasoactive neurotransmitters in fMRI signal generation are poorly understood.2-4 Fast-scan cyclic voltammetry (FSCV) quantifies electroactive neurotransmitters and oxygen concentrations with high spatiotemporal resolution by oxidizing and/or reducing analytes at a microelectrode.5,6 The additional context of evoked neurotransmitter release and higher resolution oxygen measurements makes FSCV an ideal complement to BOLD. Presently, technical limitations have stymied simultaneous FSCV/fMRI development beyond proof-of-concept.7,8 The present study addresses these limitations by providing: 1) an experimental setting optimized for simultaneous FSCV/fMRI recordings, 2) a custom hardware and software filtering setup that diminishes gradient-induced electromagnetic noise without impacting FSCV sensitivity, and 3) an in vivo demonstration of simultaneous FSCV/BOLD fMRI recording evoked oxygen changes. This optimized methodology can quantify evoked neurotransmitter release and more sensitive tissue oxygen changes during fMRI, which will enhance our ability to interpret functional neuroimaging data.Methods
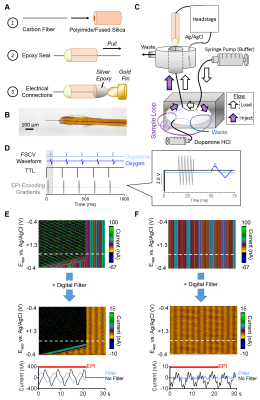
Carbon-fiber microelectrodes were fabricated using fused-silica/polyimide capillaries (Fig.1A,B).5 A flow injection setup was adapted for use inside the MR bore (Fig.1C).9 A syringe pump flowed phosphate/saline buffer (pH=7.4) to the microelectrode at 2.0mL/min, and dopamine HCl boluses were injected via 6-port injector valve (Fig.1C). FSCV data was obtained and analyzed with custom-designed High-Definition Cyclic Voltammetry software and instrumentation.10 A dopamine-sensing waveform (-0.4V to +1.3V, then back to -0.4V versus Ag/AgCl at 400 V/s) was applied at 5Hz (Fig.1D).5 Dopamine solutions were injected in triplicate, in random concentration order. FSCV data collection was synchronized with per-RF-excitation MR TTLs, delayed to avoid the gradient-encoding artifacts from single-shot EPI sequences (see below), filtered, and background-subtracted (Fig.1E,F). Principal component analysis extracted analyte currents for analysis.11
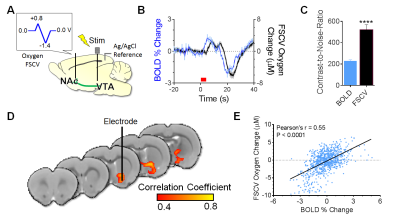
An oxygen-sensing waveform (0 to +0.8V, then to –1.4V, then back to 0V at 200 V/s) was applied at 5Hz (Fig.1D) in a rat brain.6 A twisted tungsten stimulating electrode was implanted near the ventral tegmental area (VTA) to evoke oxygen changes near the nucleus accumbens (NAc) (4s at 60Hz, pulse width=2ms). A carbon-fiber microelectrode and an Ag/AgCl reference were implanted in the ipsilateral NAc and contralateral cortex, respectively. BOLD fMRI was acquired simultaneously (TR/TE=1000/15ms, matrix=80x80, FOV=2.56cm2, 5 slices, thickness=1.0mm). FSCV time-courses were decimated by a factor of 5 to match BOLD time-scales and assess the correlation between evoked measurements. Contrast-to-noise ratios were calculated as [(Oxygen Peak Amplitude-Average Baseline)/Standard Deviation]*100.
Results and Discussion
To assess whether simultaneous FSCV/fMRI data could be collected, we synchronized voltammetry data collection to MR TTL outputs. The encoding gradient caused significant electromagnetic interference, especially with the additional 50 kHz gradient amplifier switching frequency noise. The latter was mostly removed with a digital Bessel filter (Fig.1E,F).10 A 50ms wait time was introduced to interleave the encoding gradient and FSCV waveforms, which eliminated the MR interference (Fig.1D,F). Thus, FSCV and fMRI data can be collected concurrently when the gradient artifact and waveforms are interleaved.
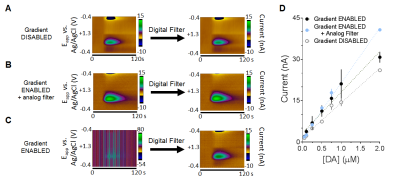
To test whether physiologically relevant concentrations of electroactive neurotransmitters can be detected using FSCV inside an MR bore, bolus injections of dopamine were introduced. With the gradient disabled, no gradient amplifier noise was present and concentrations ≥50nM could be detected (Fig.2A). With the gradient enabled, concentrations >100nM could be detected after digital filtering (Fig.2B,D). When the input signal was sent through a 2 kHz low-pass analog filter,12 concentrations ≥50 nM could be detected (Fig.2C,D). All calibration constants were >10nA/µM, consistent with the sensitivity of in vitro electrodes in a shielded environment.
With timing and filtering optimized, we electrically stimulated the VTA to evoke oxygen changes in the NAc (Fig.3A). Both evoked oxygen changes scaled with stimulation intensity (Fig.3B); however, a temporal offset was observed that may be attributed to the mismatched fMRI ROI size versus the microelectrode sampling volume and the different blood-versus-tissue oxygen diffusion therein. In addition to having 5x higher temporal resolution, FSCV had superior contrast-to-noise ratios (Fig.3C). After downsampling FSCV time-courses to match BOLD temporal resolution, we performed an unbiased voxel-wise correlation analysis between them and found strong, significant correlations near the microelectrode, the nearby NAc, and ventral pallidum (Fig.3D). When corrected for the slight diffusion shift, the oxygen metrics significantly correlated (Fig.3E). The agreement between changes in evoked tissue oxygen timing and amplitudes using simultaneous FSCV/fMRI lends credence to the functionality of our experimental setup.
Conclusion
We optimized a simultaneous FSCV and MRI experimental setup for in vitro and in vivo use. FSCV is faster, more sensitive, and can detect electroactive neurotransmitters, but BOLD provides unbiased, brain-wide measurements. This multimodality holds great promise for BOLD noise removal and improving our current understanding of fMRI data with neurochemical context at multiple temporal and spatial scales.Acknowledgements
We thank the Shih lab members for their helpful discussions and critiques. We acknowledge the help of Philip Summers for 3D modeling the electrode holder prototype. Our team is supported by NIMH R01MH111429, R41MH113252, R21 MH106939, NINDS R01NS091236, NIAAA U01AA020023, R01AA025582, NICHD U54HD079124, American Heart Association 15SDG23260025, and Brain & Behavior Research Foundation.References
1. Hillman EM. Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci 37, 161-181 (2014).
2. Raichle ME, Hartman BK, Eichling JO & Sharpe LG. Central noradrenergic regulation of cerebral blood flow and vascular permeability. Proc Natl Acad Sci U S A 72, 3726-3730 (1975).
3. Darby JM, Yonas H, Marks EC, Durham S, Snyder RW & Nemoto EM. Acute cerebral blood flow response to dopamine-induced hypertension after subarachnoid hemorrhage. J Neurosurg 80, 857-864 (1994).
4. Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA & Newman EA. Glial and neuronal control of brain blood flow. Nature 468, 232-243 (2010).
5. Rodeberg N, Sandberg S, Johnson J, Phillips PEM, Wightman RM. Hitchhiker’s Guide to Voltammetry: Acute and Chronic Electrodes for in Vivo Fast-Scan Cyclic Voltammetry. ACS Chem Neurosci 8(2), 221-234 (2017).
6. Zimmerman J, Wightman RM. Simultaneous electrochemical measurements of oxygen and dopamine in vivo. Anal Chem 63(1), 24-28 (1991).
7. Kimble CJ, Johnson DM, Winter BA, Whitlock SV, Kressin KR, Horne AE, Robinson JC, Bledsoe JM, Tye SJ, Chang SY, Agnesi F, Griessenauer CJ, Covey D, Shon YM, Bennet KE, Garris PA, Lee KH. Wireless instantaneous neurotransmitter concentration sensing system (WINCS) for intraoperative neurochemical monitoring. In Engineering in Medicine and Biology Society, 2009. EMBC 2009. Annual International Conference of the IEEE. 4856-4859 (2009).
8. Lowry JP, Griffin K, McHugh SB, Lowe AS, Tricklebank M, Sibson NR. Real-time electrochemical monitoring of brain tissue oxygen: a surrogate for functional magnetic resonance imaging in rodents. Neuroimage 52(2), 549-555 (2010).
9. Phillips PEM, Robinson DL, Stuber GD, Carelli RM, Wightman RM. Real-time measurements of phasic changes in extracellular dopamine concentration in freely moving rats by fast-scan cyclic voltammetry. Methods Mol Med 79, 443-464 (2003).
10. Bucher ES, Brooks K, Verber MD, Keithley RB, Owesson-White C, Carroll S, Takmakov P, McKinney CJ, Wightman RM. Flexible software platform for fast-scan cyclic voltammetry data acquisition and analysis. Anal Chem 85(21), 10344-53 (2013).
11. Rodeberg NT, Johnson JA, Cameron CM, Saddoris MP, Carelli RM, Wightman RM. Construction of training sets for valid calibration of in vivo cyclic voltammetric data by principal component analysis. Anal Chem 87(22), 11484-91 (2015).
12. Takmakov P, Zachek MK, Keithley RB, Walsh PL, Donley C, McCarty GS, Wightman RM. Carbon Microelectrodes with a Renewable Surface. Anal Chem 82(5), 2020-2028 (2010).
Figures