1109
Brain-wide functional organization of the hippocampus along the dorsoventral axis: an optogenetic fMRI study1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China, 3Neurology and Neurological Sciences, Stanford University, Stanford, CA, United States
Synopsis
Hippocampus plays a prominent role in central nervous system functions. It receives convergent projections and sends reciprocal divergent projections, forming an interactive cortico-hippocampal-cortical network. However, the precise brain-wide functional organization of different hippocampal activities along dorsoventral axis remains unknown. Using optogenetic fMRI, we revealed that functional organization of low frequency hippocampal activities along dorsoventral axis exhibits a gradual change from regions mainly involved in cognition and sensory processing to regions also involved in motor control and anxiety-related behavior. Additionally, hippocampal activities generally transit from long-range propagation to downstream cortical/subcortical regions to local intra-hippocampal propagation with increasing frequencies.
Purpose
Hippocampus (HP) plays a prominent role in central nervous system functions such as learning1-3, memory4,5, sensory processing6, spatial navigation7,8, motor control9,10, emotions11,12 and stress13. The HP, including the dentate gyrus (DG), can evoke large-scale influences on cortical and subcortical activities, since HP receives convergent projections from sensory and limbic regions before sending reciprocal divergent projections to form a highly interactive cortico-hippocampal-cortical network and subcortico-hippocampal-subcortical network14,15. At present, the precise functional role of these projections remains a topic of much debate. Previous anatomical and electrophysiological studies revealed that hippocampal connectivities with entorhinal cortex14,16 and amygdala17 show dorsoventral topographical gradients, indicating multiple functional gradients along the dorsoventral axis18,19. Meanwhile, gene expression studies20,21 also demonstrated multiple genetic domains along the axis, signifying discrete functional domains18,19. Our recent fMRI study demonstrated that low frequency (1 Hz), but not high frequency (40 Hz), optogenetic stimulation of excitatory neurons in anterior dorsal DG (adDG) evoked robust cortical and subcortical brain-wide fMRI responses22. However, how hippocampal activities are functionally and dynamically organized along the dorsoventral axis remains largely unknown. Since dentate gyrus (DG) primarily receives cortical and subcortical terminal projections and acts as a bridge for cortico-hippocampal-cortical and subcortico-hippocampal-subcortical communication, we examined how different frequencies of hippocampal activity initiated in DG along dorsoventral axis contribute to brain-wide functional organization by combining optogenetic23, cell-specific stimulation of CaMKIIα-expressing excitatory neurons and large-scale fMRI detection22,24,25.Materials and Methods
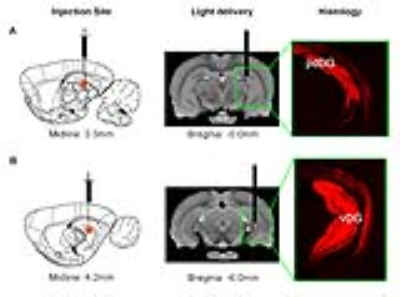
Animal preparation: As illustrated in Fig. 1, AAV5-CaMKIIα::ChR2(H134R)-mCherry was injected to posterior dorsal DG (pdDG) (n=5) or ventral DG (vDG) (n=6) of adult male SD rats. After 4-weeks, an optical fiber was implanted to deliver optogenetic stimulation.
Optogenetic stimulation: Blue light (473nm; 40mW/mm2; 1Hz, 5Hz, 10Hz, 20Hz and 40Hz) was used to determine frequency-dependent spatiotemporal characteristics of evoked pdDG and vDG responses. Two-block design of 20s light-on and 140s light-off was applied.
Optogenetic fMRI acquisition and analysis: fMRI was performed on a Bruker 7T scanner using GE-EPI (FOV=32×32mm2, matrix=64×64, α=56°, TE/TR=20/1000ms). Standard preprocessing and GLM analysis were applied to identify BOLD responses (P<0.001). BOLD signal profiles were then extracted from anatomically defined regions.
Results
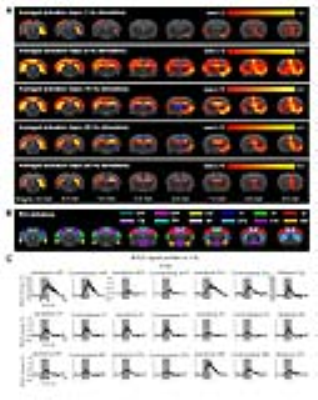
Anatomical MRI scans and histology confirmed viral injection, fiber implantation, and ChR2-mCherry expression in pdDG and vDG (Fig. 1). pdDG 1Hz stimulation evoked positive responses in ipsilateral dorsal HP (dHP) and ventral HP (vHP), bilateral primary visual cortex (V1), primary auditory cortex (A1) and cingulate cortex (Cg), while 5Hz and 10Hz evoked robust activations in bilateral vHP, dHP, entorhinal cortex (Ent), V1, A1, primary somatosensory cortex (S1), Cg, retrosplenial cortex (RS), primary motor cortex (M1), caudate putamen (CPu) and amygdala (AM). We also stimulated pdDG at 20Hz and 40Hz. Strong responses were observed in bilateral hippocampus (Fig. 2).
vDG 5Hz and 10Hz stimulation evoked robust positive responses in bilateral vHP, dHP, Ent, V1, A1, S1, Cg, RS, M1, CPu, AM and hypothalamus (hTh), which are regions related to learning, memory, sensory processing, motor control, emotions and stress. These cortical and subcortical responses decreased at 20Hz and 40Hz, while strong responses retained in vHP and dHP (Fig. 3). Note that no evoked responses were observed in naïve animal (data not shown), indicating that the observed responses were a direct result of optogenetic stimulation, not the heat-induced artifacts26.
Discussions and Conclusion
In this study, stimulations of pdDG (Fig. 2) revealed a shift from regions actively involved in only cognition and sensory processing (1Hz) to regions also associated with motor control and anxiety-related behavior (5-10Hz), and then to local hippocampal regions (20-40Hz). For vDG stimulations (Fig. 3), spatiotemporal response properties shifted from regions actively involved in cognition, sensory processing, motor control and anxiety-related behavior (5-10Hz) to local hippocampal regions (5-40Hz). Our recent study demonstrated that both BOLD response upon anterior dorsal dentate gyrus (adDG) stimulations shifted from regions actively involved in cognition and sensory processing (1Hz) to local hippocampal regions (40Hz)22.
Together, our optogenetic fMRI findings here clearly revealed that, along the dorsoventral axis (i.e., from adDG, pdDG to vDG), the downstream targets of the low frequency hippocampal activities (1-10Hz) shift from regions only actively involved in cognition and sensory processing to regions also associated with motor control and anxiety-related behavior. Furthermore, with increasing frequency, hippocampal activities generally transit from long-range propagation to downstream cortical/subcortical regions to local intra-hippocampal propagation. These results contribute to our present understanding of hippocampal activities are functionally and dynamically organized along the dorsoventral axis.
Acknowledgements
This work was supported by the Hong Kong Research Grant Council (Grants C7048-16G and HKU17103015 to E.X.W.).References
- Moser, E., Moser, M. B. & Andersen, P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. The Journal of neuroscience : the official journal of the Society for Neuroscience 13, 3916-3925 (1993).
- Moser, M. B., Moser, E. I., Forrest, E., Andersen, P. & Morris, R. G. Spatial learning with a minislab in the dorsal hippocampus. Proceedings of the National Academy of Sciences of the United States of America 92, 9697-9701 (1995).
- Fanselow, M. S. & LeDoux, J. E. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23, 229-232 (1999).
- Scoville, W. B. & Milner, B. Loss of recent memory after bilateral hippocampal lesions. Journal of neurology, neurosurgery, and psychiatry 20, 11-21 (1957).
- Squire, L. R. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological review 99, 195-231 (1992).
- Mohedano-Moriano, A. et al. Convergence of unimodal and polymodal sensory input to the entorhinal cortex in the fascicularis monkey. Neuroscience 151, 255-271, doi:10.1016/j.neuroscience.2007.09.074 (2008).
- Buzsaki, G. & Moser, E. I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature neuroscience 16, 130-138, doi:10.1038/nn.3304 (2013).
- Maguire, E. A. et al. Knowing where and getting there: a human navigation network. Science 280, 921-924 (1998).
- Vanderwolf, C. H. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalography and clinical neurophysiology 26, 407-418 (1969).
- Thoenissen, D., Zilles, K. & Toni, I. Differential involvement of parietal and precentral regions in movement preparation and motor intention. The Journal of neuroscience : the official journal of the Society for Neuroscience 22, 9024-9034 (2002).
- Kim, J. J. & Fanselow, M. S. Modality-specific retrograde amnesia of fear. Science 256, 675-677 (1992).
- Bannerman, D. M. et al. Regional dissociations within the hippocampus--memory and anxiety. Neuroscience and biobehavioral reviews 28, 273-283, doi:10.1016/j.neubiorev.2004.03.004 (2004).
- Henke, P. G. Hippocampal pathway to the amygdala and stress ulcer development. Brain research bulletin 25, 691-695 (1990).
- van Strien, N. M., Cappaert, N. L. & Witter, M. P. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nature reviews. Neuroscience 10, 272-282, doi:10.1038/nrn2614 (2009).
- Yeckel, M. F. & Berger, T. W. Feedforward excitation of the hippocampus by afferents from the entorhinal cortex: redefinition of the role of the trisynaptic pathway. Proceedings of the National Academy of Sciences of the United States of America 87, 5832-5836 (1990).
- Dolorfo, C. L. & Amaral, D. G. Entorhinal cortex of the rat: topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. The Journal of comparative neurology 398, 25-48 (1998).
- Kishi, T., Tsumori, T., Yokota, S. & Yasui, Y. Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. The Journal of comparative neurology 496, 349-368, doi:10.1002/cne.20919 (2006).
- Fanselow, M. S. & Dong, H. W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7-19, doi:10.1016/j.neuron.2009.11.031 (2010).
- Strange, B. A., Witter, M. P., Lein, E. S. & Moser, E. I. Functional organization of the hippocampal longitudinal axis. Nature reviews. Neuroscience 15, 655-669, doi:10.1038/nrn3785 (2014).
- Thompson, C. L. et al. Genomic anatomy of the hippocampus. Neuron 60, 1010-1021, doi:10.1016/j.neuron.2008.12.008 (2008).
- Dong, H. W., Swanson, L. W., Chen, L., Fanselow, M. S. & Toga, A. W. Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proceedings of the National Academy of Sciences of the United States of America 106, 11794-11799, doi:10.1073/pnas.0812608106 (2009).
- Chan, R. W. et al. Low-frequency hippocampal-cortical activity drives brain-wide resting-state functional MRI connectivity. Proceedings of the National Academy of Sciences of the United States of America 114, E6972-E6981, doi:10.1073/pnas.1703309114 (2017).
- Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature neuroscience 8, 1263-1268, doi:10.1038/nn1525 (2005).
- Lee, J. H. et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788-792, doi:10.1038/nature09108 (2010).
- Leong, A. T. et al. Long-range projections coordinate distributed brain-wide neural activity with a specific spatiotemporal profile. Proceedings of the National Academy of Sciences of the United States of America 113, E8306-E8315, doi:10.1073/pnas.1616361113 (2016).
- Christie, I. N. et al. fMRI response to blue light delivery in the naive brain: implications for combined optogenetic fMRI studies. NeuroImage 66, 634-641, doi:10.1016/j.neuroimage.2012.10.074 (2013).
Figures