1105
Differences between treated glioblastoma and metastatic brain neoplasms revealed by non-Gaussian diffusion MRI and 18F-FET-PET1Institute of Neuroscience and Medicine 4, Research Centre Juelich, Juelich, Germany, 2Department of Neurology, Faculty of Medicine, RWTH Aachen University, Aachen, Germany, 3Center of Integrated Oncology (CIO), Universities of Cologne and Bonn, Cologne, Germany, 4JARA - BRAIN - Translational Medicine, RWTH Aachen University, Aachen, Germany
Synopsis
Metastatic brain tumours constitute the majority of all brain tumours in
Introduction
Metastatic brain tumours are increasingly common, affecting 20-40% of cancer patients and constituting the majority of all brain tumours in the adult population. Differentiation between metastases and primary brain tumours, especially high-grade gliomas, may be a diagnostic problem and is important because of differences in treatment strategies1,2 for these types of tumours. Conventional MRI is sensitive to tumour lesions, however, its specificity is not sufficiently high, as both tumour types may show similar contrast patterns in standard imaging modalities. Differentiation of metastases and high-grade gliomas becomes especially problematic for patients with unknown primary tumours. Diffusion MRI techniques are capable of providing valuable information regarding microstructural properties of the tissue due to the sensitivity of water diffusion to restrictions imposed on molecular propagation by the cellular organisation (membranes, myelin, organelles). Recently, diffusion kurtosis imaging (DKI),3 an advanced non-Gaussian diffusion MRI method, has gained attention in the assessment of primary brain tumours4,5 as it was shown to provide novel biomarkers correlating with malignancy grades. The aim of this study was to elucidate the potential of two non-Gaussian diffusion methods, namely, DKI and gamma-distribution-function imaging (GDFI)6 in the differentiation of the brain metastases and high-grade tumours. MRI measurements were combined with 18F-FET-PET7,8 to obtain information on metabolic activity of a tumour.Materials and Methods
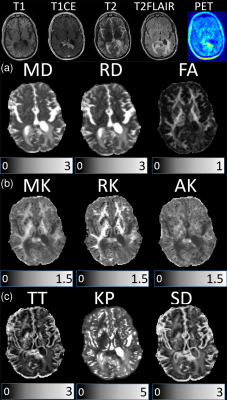
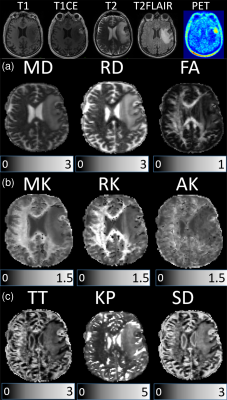
A total of 88 patients with treated brain tumours were investigated by conventional MRI, 18F-FET PET, and diffusion MRI after providing written informed consent. These patients were not recruited for a specific study but came to undergo a comprehensive examination facilitating their clinical diagnostic, treatment, and/or monitoring issues. MRI experiments were performed using a hybrid 3T PET-MR Magnetom Trio scanner (Siemens Medical System) including the following protocols: T1-weighted MPRAGE, contrast-enhanced T1-weighted MPRAGE (T1CE), T2-weighted imaging, T2-weighted FLAIR, and diffusion-weighted twice-refocused SE-EPI pulse sequence (b-values: 0, 1.0, 2.5 ms µm-2; 30 diffusion gradient directions). 18F-FET-PET was performed simultaneously with MRI using a BrainPET insert.9 Thirty-eight adult patients with high-grade gliomas (HGG) and 8 patients with 10 metastatic lesions in total (MET) were included in this study. Only metabolically active (based on 18F-FET-PET) tumours were included. Maps were computed as described elsewhere6 for the following parameters: mean/axial/radial diffusivity (MD/AD/RD), fractional/kurtosis anisotropy (FA/KA), mean/axial/radial kurtosis (MK/AK/RK), theta/kappa (TT/KP) from GDFI6, and tortuosity (TORT) from the white matter model10. The data from the different modalities were co-registered.Results and Discussion
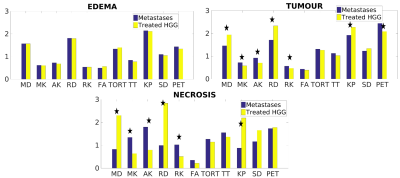
Examples of anatomic MRI, 18F-FET-PET images, and DKI/GDFI parameter maps are shown for one patient from each of the HGG and MET groups in Figures 1 and 2, respectively. They reveal pronounced spatial heterogeneity with different contrast patterns in different images/parameter maps. Such heterogeneity was characteristic for most of tumours. We developed an optimized procedure based on several imaging modalities for segmenting the lesions in three tissue types, that is, oedema (based on T2-FLAIR), necrosis (based on T1 and T1CE), and (enhancing) tumour (based on T1CE) region. Mean DKI/GDFI parameter values were evaluated and analysed separately for these regions. Significant (p<0.05) between-group differences under the Wilcoxon rank-sum test were observed for MD, RD, MK, AK, RK and KP in both tumour and necrosis regions, but not in oedema (Figure 3). The effect size of between-group differences was quantified in terms Cohen’s d values. In tumour regions, large Cohen’s d values (>0.8) were observed in MD, RD, MK, and AK with the largest value, d=1.0, found in MD. In necrosis regions, Cohen’s d values were large in MD, RD, FA, MK, AK, and KP, with the largest d=3.21 in AK. In oedema regions, only small to moderate Cohen’s d values were found with the largest d=0.48 in TT. We also evaluated relative differences for mean DKI/GDFI parameter values (as well as p-values) between the indicated tissue types and normal appearing white matter (NAWM) regions in both patient groups. The best differentiation of tumour (or oedema) region from NAWM in both groups was found with RD (or KP).
Challenges in neurooncology require clinical implementation of quantitative, biology-driven neuroimaging methodologies helping to select and develop appropriate therapies and to monitor treatment progress or failure over time. This work is contributing to the development of such methodologies by combining complex information on structural abnormality from anatomic MRI, metabolic activity from 18F-FET-PET, and microstructural alterations in primary and metastatic tumours. However, more studies are required to provide a better understanding of clinically relevant biological underpinnings of various tumour types.
Conclusion
Combined MRI, 18-FET-PET, and non-Gaussian diffusion MRI metrics demonstrate a high potential for differentiation of brain metastatic and primary tumours and, generally, may help in developing more tumour-specific treatment strategies.Acknowledgements
No acknowledgement found.References
1. David O. Kamson, Sandeep Mittal, Amy Buth, et al. Differentiation of Glioblastomas from Metastatic Brain Tumors by Tryptophan Uptake and Kinetic Analysis: a Pet Study with MRI Comparison, Mol Imaging. 2013 12(5): 327–337.
2. Platta CS, Khuntia D, Mehta MP, Suh JH. Current treatment strategies for brain metastasis and complications from therapeutic techniques: a review of current literature. Am J Clin Oncol. 2010;33:398–407.
3. Jensen, J.H., Helpern, J.A., 2010. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 23, 698-710.
4. S. Van Cauter, J. Veraart, J. Sijbers, et al., Gliomas: Diffusion Kurtosis MR Imaging in Grading, Radiology: volume 263, number 2, May 2012 (radiology.rsna.org).
5. P. Raab, E. Hattingen, K. Franz, et al., Cerebral Gliomas: Diffusional Kurtosis Imaging Analysis of Microstructural Differences, Radiology: volume 254, number 3, March 2010 (radiology.rsna.org).
6. Grinberg, F., Farrher, E., Ciobanu, L., Geffroy, F., Le Bihan, D., Shah, N.J., 2014. Non-Gaussian diffusion imaging for enhanced contrast of brain tissue affected by ischemic stroke. PLoS One 9, e89225.
7. N. Galldiks, K. Langen, R. Holy, et al. Assesment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J Nucl Med 2007;48:519-527.
8. C. Filss, N. Galldiks, G. Stoffels, et al. Comparison of 18F-FET-PET and perfusion-weighted MR imaging: a PET/MR imaging hybrid study in patients with brain tumours. J Nucl Med 2014, 55:540-545.
9. H. Herzog, K. Langen, C. Weirich, et al. High-resolution BrainPET combined with simultaneous MRI. Nuklearmedizin 2011, 50:74-82.
10. Fieremans E, Jensen JH, Helpern JA. White matter characterization with diffusional kurtosis imaging. Neuroimage. 2011;58(1):177-88. doi: 10.1016/j.neuroimage.2011.06.006.
Figures