1099
Measurement of Intra-Axonal Water Diffusivity in Normal Human White Matter1Clinic for Radiology, Medical Physics, Faculty of Medicine, Medical Center - University of Freiburg, Germany, Freiburg, Germany
Synopsis
How to measure the intra-axonal diffusion coefficient in the normal human brain? This contribution reports on such a measurement performed with minimum model assumptions. The result is about $$$2\,\rm\mu m^2/ms$$$, which constructively limits the scope of acceptable biophysical models for interpretation of diffusion MRI in terms of tissue microstructure. A side finding is the width of axonal orientation distribution in the most coherent fiber bundles, which is estimated as $$$19^\circ$$$ for the standard deviation. The core of the method is the suppression of signal from water that can move in the plane transverse to the principal fiber direction.
Introduction
Biophysical modeling is the mediator between the measurable diffusion metrics and the tissue microstructure. While modeling demands knowledge of diverse microstructural parameters, parameter determination is hardly possible without modeling because of NMR-specific parameters (such as intracellular diffusivities) that can be only determined using in-vivo MRI. This makes the problem of self-consistent parameter determination highly challenging. In particular, it is known that straightforward data fitting approach does not work [1,2].
The aim of this study was to measure the intra-axonal water diffusivity in the normal human brain using as few model assumptions as possible.
Methods
The core of the method is the suppression of signal from water molecules that can move in the plane orthogonal to the principal fiber direction in voxels with highly coherent axons. This is achieved by the planar weighting with the b-matrix $$${\bf b}={\rm diag\,}(b_F,\,b_F,\,0)/2$$$ when the principal fiber direction is selected as the z-axis. The planar weighting can be thought of as shaping the native fiber orientation distribution to a narrower one (Fig.1). For such a distribution, the diffusivity parallel to the fiber is measured using either linear weighting, $$${\bf b}={\rm diag\,}(b,\,0,\,0)$$$, or isotropic weighting, $$${\bf b}={\rm diag\,}(b,\,b,\,b)/3$$$. While the isotropic measurements yields the trace of intra-axonal diffusion tensor, the contribution of its transverse components is negligible (about $$$0.002\,\rm \mu m^2/ms$$$ for $$$1\,\rm \mu m$$$ axons). Note that we rely on the absence of a noticeable amount of fully restricted water in brain white matter [3].
In more detail, the measurement consisted of (i) DTI for selecting voxels with a single principal fiber direction as described in [4] and (ii) performing planar weighting combined with the diffusivity measurement along the normal to the suppression plane, the direction, which is referred to as the measurement direction in what follows. Measurements were repeated for multiple measurement directions and strengths of planar weighting, $$$b_F$$$. The isotropic and linear diffusivities were obtained from physically the same measurement by attributing a small portion, $$$b$$$, of the planar weighting to either the diffusivity measurement or suppressing gradients, respectively.
Signal was averaged from voxels with a single principal fiber direction in which this direction was co-linear within $$$15^\circ$$$ with one of sampled measurement directions. Isotropic and linear diffusivities were calculated and represented as functions of $$$b_F$$$. Straightforwardly obtained expressions for the signal were fitted to data with the focus on well-defined fitting parameters as qualified from numerical simulations (not shown).
Results
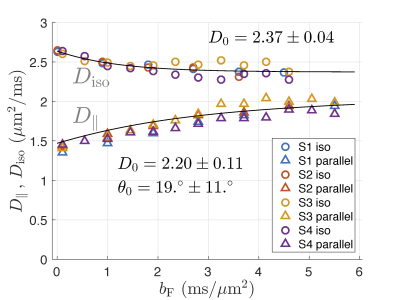
Data for all subjects are shown in Fig.2. Fitting to the pooled data for all subjects results in the intra-axonal diffusivity, $$$D_0 = 2.37\pm 0.04{\,\rm \mu m^2/ms}$$$ for isotropic measurement and $$$D_0 = 2.24\pm 0.05{\,\rm \mu m^2/ms}$$$ for linear measurement (all errors estimated from the fitting residuals). Results for individual subjects deviate slightly from so defined mean values (Fig.2). The fiber orientation distribution was approximated by Gaussian in $$$\sin\theta$$$ for small angles. Its width, $$$\sigma_0$$$, was estimated from the linear weighting and corrected for the voxel selection cone of $$$15^\circ$$$ with the result $$$\sigma_0 = 0.33\pm 0.15$$$, which corresponds to the angle $$$\theta_0 = \arcsin \sigma_0 = 19 ^\circ \pm 11 ^\circ$$$.Discussion
The values of diffusivities found in this study slightly overestimate their genuine long-time diffusion coefficients due to the wave form of the involved gradients. In more detail, the overestimation results from the finite width of the gradient power spectrum in combination with the frequency dependence of velocity correlation function in proportion to $$$\sqrt{\omega}$$$ for low frequencies, $$$\omega$$$, [5,6]. This gives a correction about $$$-0.25\,\rm \mu m^2/ms$$$ to the above results.
With this small correction, the obtained values of $$$D_0$$$ agree well with the interval $$$[1.9,\,2.2]\,\rm \mu m^2/ms$$$ found by extrapolating data from ultra-high $$$b$$$ to $$$b\to\infty$$$ [7]. The width of the fiber orientation distribution agrees well with histology [8,9] and MRI-based studies [7--9].
The values of $$$D_0$$$ from isotropic measurement is larger than that from the linear measurement by $$$0.17\pm 0.12\,\rm \mu m^2/ms$$$, which remains to be explained, possibly by model imperfections. For example, deviations of axons from straight cylinders over the water diffusion length can create apparent transverse components of intra-axonal diffusion tensor, which are then added to its trace as measured using the isotropic weighting. A simple estimate shows that the curvature with the radius $$$50\,\rm \mu m$$$ of all cylinders would explain the difference. Assigning such a curvature to all axons might be unrealistic, but a relatively large contribution from a small sub-population of cellular processes cannot be currently excluded.
The obtained value of intra-axonal diffusion coefficient constructively limits the scope of acceptable biophysical models for diffusion MRI. Beyond this role, the present results will be subjected to a closer investigation in norm and pathology in future work.
Acknowledgements
No acknowledgement found.References
[1] Ileana O Jelescu, Jelle Veraart, Els Fieremans, and Dmitry S Novikov. Degeneracy in model parameter estimation for multi-compartmental diffusion in neuronal tissue. NMR in Biomedicine,29(1):33–47, 2016.
[2] Dmitry S. Novikov, Jelle Veraart, Ileana O Jelescu, and Els Fieremans. Mapping orientationaland microstructural metrics of neuronal integrity with in vivo diffusion MRI. https://arxiv.org/abs/1609.09144.
[3] Bibek Dhital, Elias Kellner, Marco Reisert, and Valerij G. Kiselev. Isotropic diffusion weighting provides insight on diffusion compartments in human brain white matter in vivo. In Proc. 23st Annual Meeting of ISMRM, page 2788, Toronto, Canada, June 2015.
[4] Els Fieremans, Jens H Jensen, and Joseph A Helpern. White matter characterization withdiffusional kurtosis imaging. Neuroimage, 58(1):177–88, Sep 2011.
[5] Dmitry S Novikov, Jens H Jensen, Joseph A Helpern, and Els Fieremans. Revealing mesoscopicstructural universality with diffusion.Proc Natl Acad Sci U S A, 111(14):5088–93, Apr 2014.
[6] Els Fieremans, Lauren M Burcaw, Hong-Hsi Lee, Gregory Lemberskiy, Jelle Veraart, and Dmitry S Novikov. In vivo observation and biophysical interpretation of time-dependent diffusion in human white matter. Neuroimage, 129:414–27, Apr 2016.
[7] J. Veraart, E. Fieremans, and D. S. Novikov. Universal power-law scaling of water diffusion in human brain defines what we see with MRI. ArXiv e-prints, September 2016.
[8] Trygve B. Leergaard, Nathan S. White, Alex de Crespigny, Ingeborg Bolstad, Helen D’Arceuil, Jan G. Bjaalie, and Anders M. Dale. Quantitative histological validation of diffusion MRI fiber orientation distributions in the rat brain. PLOS ONE, 5(1):1–8, 01 2010.
[9] Itamar Ronen, Matthew Budde, Ece Ercan, Jacopo Annese, Aranee Techawiboonwong, and Andrew Webb. Microstructural organization of axons in the human corpus callosum quantified by diffusion-weighted magnetic resonance spectroscopy of N-acetylaspartate and post-mortem histology. Brain Struct Funct, 219(5):1773–85, Sep 2014.
Figures