1094
Biophysical modeling of the gray matter: does the “stick” model hold?1Center for Biomedical Imaging, NYU School of Medicine, New York, NY, United States, 2CUBRIC, Cardiff University, Cardiff, United Kingdom
Synopsis
The use of term “neurites” implies that many biophysical models of diffusion in the white matter can be applied in the gray matter as well. However, the validity of representing dendrites as a collection of zero-radius impermeably sticks, a widely adopted representation of myelinated axons, has not been evaluated yet. By evaluating the diffusion-weighted signal decay as a function of the $$$b$$$-value up to $$$b=25000\,\mathrm{ms/\mu\,m^2}$$$ in the living human brain, we show that a more accurate representation of the diffusion in neuronal processes in the gray matter must account for a fast proton exchange between the intra- and extra-cellular compartments.
Introduction
The gray matter is mainly composed of cell bodies, glial cells, and synapses, which are embedded in an intricately organized network of cellular processes, that are projections of the cell body that can be either dendrites or myelinated as well as unmyelinated axons$$$^1$$$. A clear differentiation between dendrites and axons is sometimes lacking, and an overarching term "neurite" is then used to refer to either of the projections.
We observe an increasing use of the term neurite instead of (myelinated) axon in the context of biophysical modeling of diffusion in the brain, thereby implying the model's applicability in both the white and gray matter$$$^{2,3}$$$. However, the overarching assumption behind compartmental models of diffusion in neuronal tissue is that the intra-cellular compartment is well represented by narrow impermeable channels ("sticks") in which the radial diffusivity is negligible$$$^{2,4}$$$. The proton exchange between intra- and extra-cellular spaces must be sufficiently slow to be considered impermeable on the time scale of the experiment.
This assumption has been validated for myelinated axons on a
state-of-the art clinical scanner by studying exchange$$$^{5,6}$$$ or by retrieving a nontrivial power law scaling
of the diffusion-weigthed signal as function of the $$$b$$$-value$$$^{7,8}$$$. Here we validate the “stick” assumptions
in the cortical gray matter while exploiting the strong gradients of the
Siemens Connectom 3T scanner to boost the SNR.
We interpret observed deviations from the power law scaling in terms of
finite neurite radii and exchange times to provide a comprehensive picture of
the challenges of translating our white matter models to the gray matter.
Data
Four volunteers were scanned on a Connectom scanner with $$$\Delta/\delta\,=\,30/13\,\mathrm{ms}$$$ and maximal gradient amplitude $$$G=289\,\mathrm{mT/m}$$$. Diffusion-weighting was applied along 60 gradient directions for a spectrum of $$$b$$$-values up to $$$25\,\mathrm{ms/\mu\,m^2}$$$. Furthermore, $$$\mathrm{TR/TE}\,=3500/62\,\mathrm{ms}$$$ and resolution $$$3\,\times\,3\times\,3\,\mathrm{mm}^3$$$. Data was denoised9, gibbs10, eddy current11, Rician bias corrected12, and isotropically-averaged per $$$b$$$-shell to cancel the orientation distribution function.
Methods
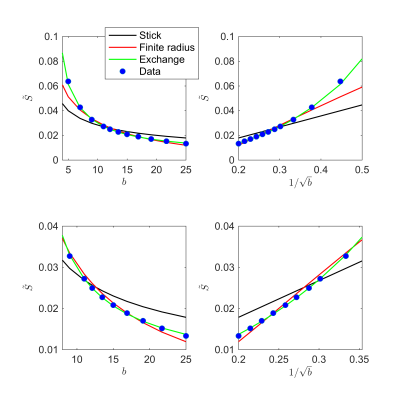
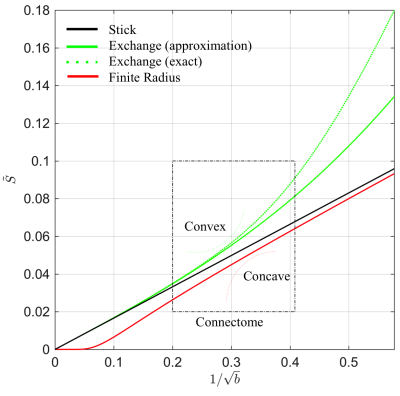
In many biophysical models, the intra-cellular compartment is represented by an array of zero-radius, impermeable “sticks” inside which diffusion is locally one-dimensional, i.e. radial intra-cellular diffusivity $$$D_c^\perp\,\equiv\,0$$$2,3. The stick model yields an intra-cellular signal decay $$$\tilde{S}$$$ that asymptotically scales according to a power law with exponent $$$\alpha=\,1/2$$$: $$ \tilde{S}(b)\,\simeq\,\beta\,b^{-\alpha }\,+\,\gamma\quad\mathrm{for}\quad\,bD_c^\parallel\gg1,\quad\,[1]$$ with $$$\gamma$$$ a immobile water fraction and $$$\beta$$$ a scaling factor that depends on the intra-cellular signal fraction7,8. This decay only holds in the absence of any exponentially fast decaying extra-axonal signal. Therefore, we restrict our analysis to $$$b>6\,\mathrm{ms/\mu\,m^2}$$$. Sensitivity of MR to either finite neurite radius or a notable exchange rate between different compartments would break the $$$b^{-1/2}$$$-scaling:
1. Finite neurite radius: A finite $$$D_c^\perp > 0$$$ results in a truncated power law: $$\tilde{S}(b)\,\simeq\,\beta\,e^{-bD_c^\perp}b^{-1/2}\,+\,\gamma.\quad\,[2]$$
2. Exchange: The isotropic averaging of a two-compartment anisotropic Kärger model13 with exchange rate$$$\,r>0$$$ yields asymptotic signal decay:$$\tilde{S}(b)\,\simeq\,\beta\left(\,b^{-1/2}+2\frac{rt}{D_e^\perp}b^{-3/2}\right)+\gamma,\quad\,[3]$$ with $$$D_e^\perp$$$ the radial diffusivity in the interstitial space and $$$t=T_\mathrm{E}$$$ the time during which exchange happens.
We evaluate the signal decay as function of the
$$$b$$$-value and study the impact of accounting for non-zero radii, exchange
rates, and/or immobile water fraction qualitatively. We also assess the goodness-of-fit in comparison to the
power law that reflects the ideal "stick" model using the corrected
Akaike Information Criterion14 (AICc) in all voxels of the cortical gray matter
that were segmented using FreeSurfer15.
Results
Fig. 1 shows the signal decay, averaged over all cortical GM voxels, as a function of $$$b$$$. The non-linear scaling of the signal as a function of $$$1/\sqrt(b)$$$ indicates deviations from the "stick" model.
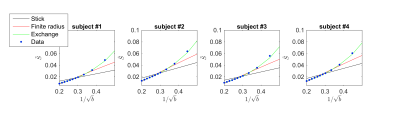
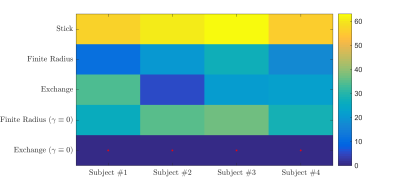
The exchange model ($$$\gamma \equiv 0$$$) fits the data best in all subjects, qualitatively (Fig. 2) and statistically (Fig 3). The average residence times, $$$1/r$$$ vary from approximately 10-15ms or 20-30ms if we assume $$$D_e^\perp\,=\,1\,\mathrm{\mu\,m^2/ms}$$$ or $$$D_e^\perp\,=\,0.5\,\mathrm{\mu\,m^2/ms}$$$, respectively.
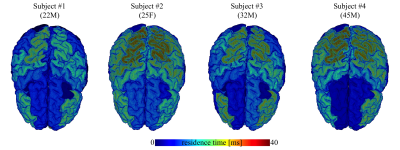
In Fig. 4, we show the average residence times, computed within the different cortical areas, and map them on the three-dimensional rendering of a single brain for anatomical reference. All subjects demonstrate spatial variability of the residence times, but overall -considering the precision of the measurement- they show good mutual correspondence.
Discussion and Conclusions
By observing significant deviations from the power law scaling, we uncovered deviations from the stick model in the gray matter. Biophysical models that are building upon that assumption may need to be interpreted with care when applied in the gray matter.
Evaluation of models of exchange and finite radii suggests that neurites are in notable exchange with another compartment in the gray matter. The measured exchange might result from actual membrane permeability. However the significantly lower residence times in comparison with previous studies16,17 might reflect apparent exchange due to protons temporarily residing in the multitude of dendritic spines18. Monte-Carlo simulations and model refinements will address this pending question.
Acknowledgements
JV is a Postdoctoral Fellow of the Research Foundation - Flanders (FWO; grant number 12S1615N). EF and DSN were supported by the NIH/NINDS award R01NS088040. The data were partially acquired at the UK National Facility for In Vivo MR Imaging of Human Tissue Microstructure funded by the EPSRC (grant EP/M029778/1), and The Wolfson Foundation. DKJ is supported by a Wellcome Trust Investigator Award (096646/Z/11/Z) and a Wellcome Trust Strategic Award (104943/Z/14/Z).References
1. H. Gray. Gray's Anatomy, 30th edition. Philadelphia: Lippincott Williams & Wilkins (1985).
2. S.N. Jespersen, C.D. Kroenke, L. Østergaard, J.J.H. Ackerman, D.A. Yablonskiy, Modeling dendrite density from magnetic resonance diffusion measurements, NeuroImage 34(4), 1473-1486 (2007).
3. H. Zhang, T. Schneider, C.A. Wheeler-Kingshott, and D.C. Alexander, NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain, Neuroimage, 61(4), 1000-1016 (2012).
4. C. D. Kroenke, J. J. Ackerman, and D. A. Yablonskiy, On the nature of the NAA diffusion attenuated MR signal in the central nervous system, Magnetic resonance in medicine 52, 1052–1059 (2004).
5. M. Nilsson, J. Latt, D. van Westen, S. Brockstedt, S. Lasic, F. Stahlberg, and D. Topgaard. Noninvasive mapping of water diffusional exchange in the human brain using filter-exchange imaging, Magnetic Resonance in Medicine 69,1573–1581 (2013).
6. M. Nilsson, J. Latt, E. Nordh, R. Wirestam, F. Stahlberg, and S. Brockstedt. On the effects of a varied diffusion time in vivo: is the diffusion in white matter restricted? Magnetic Resonance Imaging 27:176–187 (2009).
7. J. Veraart, E. Fieremans, D.S. Novikov , Universal power-law scaling of water diffusion in human brain defines what we see with MRI. arXiv:1609.09145 (2016).
8. E. T. McKinnon, J. H. Jensen, G. R. Glenn, and J. A. Helpern, “Dependence on b-value of the direction-averaged diffusion-weighted imaging signal in brain,” Magnetic resonance imaging 36, 121–127 (2017).
9. J. Veraart, D.S. Novikov, D. Christiaens, B. Ades-Aron, J. Sijbers, and E. Fieremans, Denoising of diffusion MRI using random matrix theory. NeuroImage, 142, 394-406 (2016).
10. E. Kellner, B. Dhital, V. G. Kiselev, and M. Reisert, “Gibbs-ringing artifact removal based on local subvoxel-shifts,” Magnetic resonance in medicine 76, 1574–1581 (2016).
11. J. L. Andersson and S. N. Sotiropoulos, “An integrated approach to correction for off-resonance effects and subject movement in diffu-sion MR imaging,” Neuroimage 125, 1063–1078 (2016).
12. C. G. Koay and P. J. Basser, “Analytically exact correction scheme for signal extraction from noisy magnitude MR signals,” Journal of Magnetic Resonance 179, 317–322 (2006).
13. J. Kärger, NMR self-diffusion studies in heterogeneous systems. Adv. Colloid. Interfac,23:129–148 (1985).
14. K. P. Burnham and D. R. Anderson, “Information and Likelihood Theory: A Basis for Model Selection and Inference,” in Model Selection and Multimodel Inference (Springer New York, 2002) pp. 49–97.
15. A.M. Dale, B. Fischl, M.I. Sereno, Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179-194 (1999).
16. J.D. Quirk, G.L.Bretthorst, T.Q. Duong, A.Z. Snyder, C.S. Springer, J.J.H. Ackerman, and J.J. Neil, Equilibrium water exchange between the intra and extracellular spaces of mammalian brain. Magnetic Resonance in Medicine 50:493–499 (2003).
17. D.M. Yang, J.E. Huettner, G.L. Bretthorst, J.J. Neil, J.R. Garbow, and J.J.H. Ackerman, Intracellular water preexchange lifetime in neurons and astrocytes. Magnetic Resonance in Medicine, doi:10.1002/mrm.26781 (2017).
16. E.G. Gray, "Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex", Nature 183: 1592–1593. (1959).
Figures