1089
In vivo MRI detection of early β-amyloid pathologies targeted by curcumin-conjugated magnetic nanoparticles1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China
Synopsis
Early state β-amyloid oligomers (AβOs) and
Purpose
Both early stage soluble Aβ oligomers (AβOs) and late stage insoluble Aβ plaques are the pathological hallmarks of Alzheimer's disease (AD) brains1. During AD progression, a gradual shift in predominance occurs from the more neurotoxic soluble AβOs in the early stages to insoluble Aβ plaques2,3. Most of the existing molecular MRI approaches are limited by the poor sensitivity for early Aβ pathology detection or contrast agent toxicity4-8. Curcumin, a natural, safe product extracted from turmeric, possesses the ability to bind to Aβ species9. We have recently synthesized a new curcumin-conjugated magnetic nanoparticles (Cur-MNPs) to target the Aβ pathologies with low cytotoxicity and excellent blood-brain-barrier (BBB) penetration pototential10. In this preliminary study, we aimed to assess this novel Cur-MNPs based MRI contrast agent for its in vivo ability to detect and visualize the early and late Aβ pathologies in transgenic AD mice.Methods
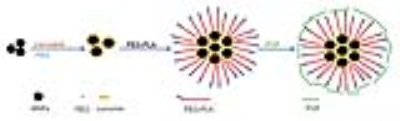
Cur-MNPs: A novel curcumin-conjugated superparamagnetic iron oxide (SPIO) stabilized by amphiphilic block copolymer was designed and characterized in this study. The hydrodynamic size of Cur-MNPs was ~90nm. The core consisted of several individual MNPs and curcumin conjugated on their surface. The outer layer was a coating of polyethylene glycol-polylactic acid block copolymer (PEG-PLA) and polyvinylpyrrolidone (PVP) (Figure 1).
Animal groups and preparation: Four groups of mice were used in this study: 4 3-mon-old 5xFAD mice; 3 3-mon-old wild type mice as controls; 4 10-mon-old 5xFAD mice; and 4 10-mon-old wild type mice as controls. Note that both young and old transgenic 5xFAD AD mice were chosen here because they are known to manifest the very early and late stage of Aβ pathologies, respectively. Animals were injected with Cur-MNPs intravenously via tail vein (0.315mmol Fe/kg in 150μl) and scanned by MRI four hours after injection. They were anesthetized under 1.5% isoflurane mixed with 90% oxygen during MRI scan.
Data acquisition and analysis: MRI data was acquired on a Bruker 7T preclinical scanner using a 2D GRE sequence with TR/TE=3000/12.3ms, FA=80.1o, bandwidth=25kHz, FOV=25.6mm×25.6mm, matrix size=512×512, in-plane resolution=50μm×50μm, slice thickness=0.38mm with 0.02mm gap, 35 slices, two repetitions, and total acquisition time of 51min. Two image datasets reconstructed from the two repetitions were realigned before averaging to reduce the effect of animal motion artifacts on high-resolution image quality.
Results
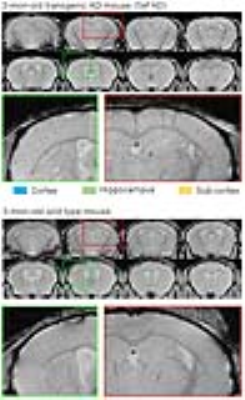
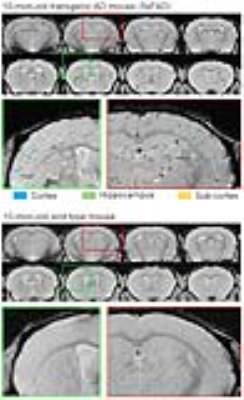
Figure 2 presents the serial in vivo T2*-weighted images of one 3-mon-old (young) 5xFAD mouse and one age-matched wild type control after intravenous Cur-MNPs injection. Hypointense spots were typically observed in the 5xFAD mice but not in the controls. These results directly indicate the capability of Cur-MNPs in detecting Aβ pathologies during the early stage of AD progress. Likely, Cur-MNPs here targeted AβOs rather than Aβ plaques because the 5xFAD mice are known to predominantly exhibit AβOs at 3 month11. Figure 3 shows the in vivo T2*-weighted images of one 10-mon-old (old) 5xFAD transgenic mouse and one control. In contrast, these hypointense spots in the old 5xFAD mice were generally denser and larger as expected because 10-mon-old 5xFAD mice have significantly increased presence of Aβ pathologies, and they are dominated by the Aβ plaques11. Direct and detailed histological validation of specific targeting of various forms of Aβ pathologies (both soluble AβOs and insoluable Aβ plaques) is presently underway.Discussion and Conclusion
This preliminary study demonstrated for the first time that Cur-MNPs could detect Aβ pathologies in vivo (including AβOs and Aβ plaques) during both early and late stage of AD progression in transgenic AD mice. Curcumin possesses the ability to bind to Aβ species and naturally binds to the SPIO without chemical linkers9,10,12. Moreover, in contrast to the Aβ peptide that was previously used to conjugate iron nanoparticles, curcumin safety has been demonstrated in our human clinical trial13. Thus, curcumin Aβ binding presents an excellent mechanism for targeting Aβ pathologies in AD brains. Our MRI findings show that old mice exhibit more Aβ pathologies than young mice, demonstrating that our molecular MRI approach is applicable to both early and late stage of AD. Although Aβ deposition can begin as early as at 1.5 months in 5xFAD mice, spatial memory loss only occurs at 4-5 months11. This indicates that our technique can detect Aβ pathologies before the cognitive decline occurs.Acknowledgements
This work was supported by the Hong Kong Research Grant Council (HKU17115116 to E.X.W.).References
1.Adlard, P.A., et al. A review of beta-amyloid neuroimaging in Alzheimer's disease. Frontiers in neuroscience 8, 327 (2014).
2.Jack, C.R., Jr. & Holtzman, D.M. Biomarker modeling of Alzheimer's disease. Neuron 80, 1347-1358 (2013).
3.Wisniewski, T. & Goni, F. Immunotherapeutic approaches for Alzheimer's disease. Neuron 85, 1162-1176 (2015).
4.Poduslo, J.F., et al. Molecular targeting of Alzheimer's amyloid plaques for contrast-enhanced magnetic resonance imaging. Neurobiology of disease 11, 315-329 (2002).
5.Wadghiri, Y.Z., et al. Detection of Alzheimer's amyloid in transgenic mice using magnetic resonance microimaging. Magnetic resonance in medicine 50, 293-302 (2003).
6.Sigurdsson, E.M., et al. A non-toxic ligand for voxel-based MRI analysis of plaques in AD transgenic mice. Neurobiology of aging 29, 836-847 (2008).
7.Yang, J., et al. Detection of amyloid plaques targeted by USPIO-Aβ1–42 in Alzheimer's disease transgenic mice using magnetic resonance microimaging. Neuroimage 55, 1600-1609 (2011).
8.Braakman, N., et al. Longitudinal assessment of Alzheimer's β‐amyloid plaque development in transgenic mice monitored by in vivo magnetic resonance microimaging. Journal of magnetic resonance imaging 24, 530-536 (2006).
9.Mishra, S. & Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer's disease: An overview. Annals of Indian Academy of Neurology 11, 13 (2008).
10.Cheng, K.K., et al. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer's disease mice using magnetic resonance imaging (MRI). Biomaterials 44, 155-172 (2015).
11.Oakley, H., et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 10129-10140 (2006).
12.Tripathi, S. Development of curcumin loaded-magnetic iron oxide nanoparticles. (2015). 13. Baum, L., et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. Journal of clinical psychopharmacology 28, 110-113 (2008).
Figures