1087
Creatine kinase energy supply correlates with mechanical work and efficiency in healthy and failing human heart: a combined noninvasive MRI/MRS study1Division of MR Research, Department of Radiology, The Johns Hopkins University, Baltimore, MD, United States, 2Department of Diagnostic and Interventional Imaging, University of Texas Health Science Center at Houston, Houston, TX, United States, 3Systems and Biomedical Engineering, Faculty of Engineering, Cairo University, Cairo, Egypt, 4Department of Cardiology, The Johns Hopkins University, Baltimore, MD, United States
Synopsis
It is hypothesized that chemical energy supply is insufficient to fuel normal mechanical pump function in heart failure (HF). To test whether reduced function correlates with reduced energy supply, we used magnetic resonance spectroscopy and imaging to measure adenosine triphosphate (ATP) synthesis via the creatine kinase reaction–the heart’s primary reserve–and cardiac mechanical stroke work in 14 healthy subjects and 27 patients with mild-to-moderate HF. We found significantly reduced cardiac creatine kinase flux that correlated with peak and average stroke work rates and with mechanical efficiency. These first noninvasive findings are consistent with the energy deprivation hypothesis of HF.
PURPOSE
Impaired
energy metabolism has long been hypothesized to underlie human heart failure (HF)(1-3). The adenosine triphosphate (ATP)
energy supplied by the creatine kinase (CK) reaction, the heart’s primary
reserve, is reduced in HF(4) and independently predicts HF events(5). However, the
relationship between the CK ATP supply and cardiac stroke work has never been quantified
in human hearts. This study combines noninvasive MRI and phosphorus (31P)
MRS methods to test whether reduced CK energy supply is associated with reduced
mechanical work in HF patients.
METHODS
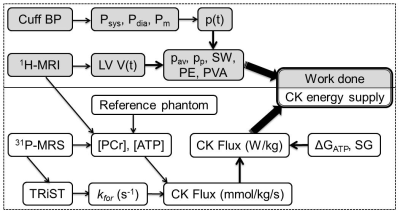
We studied 27 HF patients (NYHA class I-III) with left-ventricular (LV) ejection fractions <45% but no evidence of coronary disease, and 14 age-matched healthy subjects. A combined MRI/MRS protocol for measuring CK energy supply and cardiac work was implemented on a 3.0 Tesla Philips Achieva MRI scanner (Fig.1).
Absolute anterior LV concentrations of phosphocreatine (PCr) and ATP in mmol/kg wet wt. were measured by 31P MRS using an external concentration referencing method(6). The pseudo-first order rate constant of the CK reaction, kf , was measured using triple repetition-time saturation-transfer (TRiST) (7). The CK flux, kf [PCr] in mmol/kg/s, was converted to Watts/kg (W/kg) based on a free-energy of ATP hydrolysis, ΔGATP=60kJ/mol, determined from healthy and HF patients(4).
Cardiac work was calculated from pressure-volume (PV) loops(9-11) determined noninvasively from 30-phase cardiac cine MRI. The time-dependent LV chamber volume, V(t), and LV mass (LVM) were measured by delineating the endo- and epi-cardial contours in short-axis cine MRIs at all time points: LVM was obtained from the difference in contours and the specific gravity (=1.03). LV pressure measurements, P(t), require an LV catheter(9) which was not feasible for these subjects not undergoing catheterization. Instead, P(t) was approximated by a step function scaled to the mean brachial arterial blood pressure during ejection, assuming negligible end-diastolic pressure for the unstressed LV volume (V0).
The PV-work was calculated from w(t)= P(t)∆V(t), the stroke work (SW) by the integral of w(t) over one cardiac cycle, and the mechanical power (rate of energy consumption) by the time-derivative of w(t). The internal cardiac ‘potential energy’ (PE) was estimated from the area of the PV-curve under the end-systolic PV-line(10). The total mechanical energy (and work) was estimated by the sum, PVA=SW+PE, and the mechanical efficiency by SW/PVA(11). The average (pav) and peak (pp) cardiac power, normalized by LV mass (LVM) in W/kg, were determined and compared to the CK energy supply in W/kg in healthy and HF subjects using unpaired t-testing and regression.
RESULTS
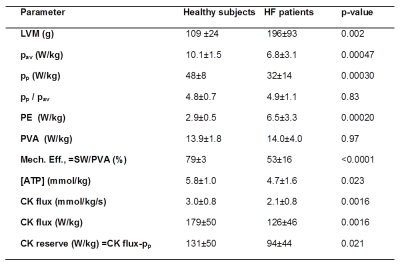
In HF patients, peak and average mechanical power are both reduced 33% compared to healthy subjects (p<0.0005;Table 1). Consequently, the ratio of the peak-to-average cardiac power is the same in both groups: about 5-fold. In HF, PE normalized by LVM was over twice that of healthy subjects, while the total PVA in W/kg was similar in both groups. Because of the higher PE, mechanical efficiency, was reduced by a third in HF patients (53% vs 79% in healthy subjects, p<0.0001).
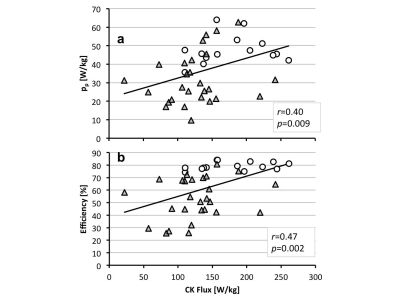
The CK energy supply was also significantly reduced by a third from 179W/kg in healthy subjects to 126W/kg in HF (p<0.002). Consequently, the difference between the resting CK energy supply and the normalized peak mechanical power, or CK energy reserve, decreased from 131W/kg in healthy subjects to 94W/kg in HF (p=0.02). Regression analysis reveals significant correlations between the CK energy supply and energy utilization (Fig.2), including the average and peak power (pav, r=0.43, p=0.004; pp, r=0.40, p=0.009), and mechanical efficiency (r=0.47; p=0.002).
DISCUSSION
This first study to noninvasively document the magnitude of the CK energy supply relative to the energy required for mechanical contraction in the same hearts shows that the CK supply in healthy hearts (179W/kg) exceeds average work rate (pav) by 18-fold at rest. However, CK supply exceeds peak demand (pp) by only 3.7-fold, which may be limiting at high work-loads if CK flux doesn’t increase with workload in healthy subjects(4,12). In HF, CK flux was significantly lower and correlated with work-rate and cardiac efficiency.
While the CK reaction provides a surplus of energy to support cardiac function in both healthy and HF subjects at rest, the reduced mechanical efficiency in HF further taxes the energy available for mechanical work. For the average HF patient studied here, the reduced CK flux (Table 1) at 53% mechanical efficiency should be able to support a doubling of peak mechanical power during stress, while maintaining a normal resting peak-to-average mechanical workload of ~5. However, patients with greater reductions in CK flux and/or lower efficiency (Fig.2b), may experience energy-related contractile decline with worsening or activity-limiting symptoms.
Acknowledgements
Supported by AHA grant 13GRNT17050100 and NIH grant R01 HL61912.References
1. Herrmann G. The Chemical Nature of Heart Failure. Ann Intern Med 1939;12:1233–44.
2. McMurray JJ, Pfeffer MA. Heart failure. Lancet 2005;365:1877–89.
3. Neubauer S. The failing heart-an engine out of fuel. N Engl J Med. 2007;356:1140-1151.
4. Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A. 2005;102:808-13.
5. Bottomley PA, Panjrath GS, Lai S, Hirsch GA, Wu K, Najjar SS, Steinberg A, Gerstenblith G, Weiss RG. Metabolic Rates of ATP Transfer Through Creatine Kinase (CK Flux) Predict Clinical Heart Failure Events and Death. Sci Transl Med. 2013;5:215re3-215re3. doi/10.1126/scitranslmed.3007328.
6. El-Sharkawy A-MM, Gabr RE, Schär M, Weiss RG, Bottomley PA. Quantification of human high-energy phosphate metabolite concentrations at 3 T with partial volume and sensitivity corrections. NMR Biomed 2013;26:1363–71.
7. Schär M, El-Sharkawy A-MM, Weiss RG, Bottomley PA. Triple repetition time saturation transfer (TRiST) 31P spectroscopy for measuring human creatine kinase reaction kinetics. Magn Reson Med 2010;63:1493–501.
8. Hirsch GA, Bottomley PA, Gerstenblith G, Weiss RG. Allopurinol Acutely Increases Adenosine Triphospate Energy Delivery in Failing Human Hearts. J Am Coll Cardiol 2012:802–8.
9. Suga H. Ventricular energetics. Physiol Rev. 1990;70:247-277.
10. Timmer SAJ, Germans T, Götte MJW, Rüssel IK, Dijkmans PA, Lubberink M, ten Berg JM, ten Cate FJ, Lammertsma AA, Knaapen P, van Rossum AC. Determinants of myocardial energetics and efficiency in symptomatic hypertrophic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2010;37:779-788.
11. Timmer SAJ, Germans T, Brouwer WP, Lubberink M, van der Velden J, Wilde AAM, Christiaans I, Lammertsma AA, Knaapen P, van Rossum AC. Carriers of the hypertrophic cardiomyopathy MYBPC3 mutation are characterized by reduced myocardial efficiency in the absence of hypertrophy and microvascular dysfunction. Eur J Heart Fail. 2011;13:1283-1289.
12. Weiss K, Bottomley PA, Weiss RG. On the theoretical limits of detecting cyclic changes in cardiac high-energy phosphates and creatine kinase reaction kinetics using in vivo 31P MRS. NMR Biomed. 2015;28:694-705.
Figures