1072
In Vivo Characterization of 3D Skull and Brain Motion using MR Elastography with Multi-Excitation Head DriverZiying Yin1, Yi Sui1, Joshua D Trzasko1, Phillip J Rossman1, Armando Manduca1, Richard L Ehman1, and John Huston III1
1Radiology, Mayo clinic, Rochester, MN, United States
Synopsis
Characterization of skull-brain interactions during applied motion is essential to understanding the mechanics of traumatic brain injury. In this study, MR elastography was performed on volunteers to study in vivo skull-brain motion responding to different vibrational directions using a multi-excitation driver. With novel dual-saturation imaging and dual-sensitivity motion encoding schemes, we directly measured relative skull-brain displacement on a voxel basis. Our results show that the skull-brain interface tends to significantly attenuate and delay rotational motion compared to translational motion. In slip interface imaging, the skull-brain slip interface is not completely evident, and the slip pattern is spatially heterogeneous.
Introduction
Characterization of skull-brain interactions during applied motion is essential to understanding the mechanics of traumatic brain injury, but in vivo characterization is limited. A previous study utilized MR elastography (MRE) combined with accelerometers to investigate the relative brain-skull motion; however, the skull motion was indirectly assessed and the motion was limited to vertical vibrations[1]. Direct measurement of skull and brain motion responding to different vibrational directions could expand our understanding of how the skull-brain interface attenuates and delays motion transmission into the brain. Towards this goal, we developed a dual-saturation imaging and dual-sensitivity motion encoding scheme to directly measure and reliably unwrap the skull as well as brain phase images using MRE. In addition we used this technique to study the relative skull-brain motion in volunteers with a newly developed multi-excitation head driver.Methods
MRE exams were performed on 3 healthy volunteers with a multi-excitation head driver that is integrated into a 12-channel head coil (Fig.1). Three distinct excitation patterns (anterior-posterior(AP), left-right(LR), and clockwise(CW)) at 60-Hz were introduced into the head by independently setting the amplitude and phase of the individual actuators (Fig.1D). Dual-saturation imaging- i.e., one MRE scan with water-selective SPSP excitation (fat-suppression) and the other with fat-selective SPSP excitation (water-suppression) – was performed to characterize mechanically-induced motion of the brain and skull respectively. The signal from the skull was detected from the fatty marrow contained within the medullary cavity. The 3D skull-plus-brain MRE data were generated by combing the dominantly water and fat MRE data in the complex image domain. MRE data was acquired with a modified SS-SE-EPI-MRE sequence incorporating the dual-sensitivity motion encoding scheme, in which the amplitude of negative MEGs were set to be smaller (77.7%) than that of positive MEGs, resulting in both low and high motion encoding sensitivity phase in a single scan when these phases are added or subtracted respectively. The low-sensitivity phase images that are practically free of skull-based phase wrap were then used to guide unwrapping of the high-sensitivity phase image. Other imaging parameters include: TR/TE=4000/58.7ms; FOV=24cm; 80×80 acquisition matrix reconstructed to 128×128; 48 contiguous 3-mm-thick axial slices; 2xASSET; 4 phase offsets. MRE displacements from the brain and skull ROIs (Fig.2D) were fitted to a model of rigid-body motion to obtain rigid-body translation and rotation. The amplitude and temporal phase delay between the skull and brain were compared for each rigid-body component. The relative motion of the skull and brain was also visualized by slip interface imaging (SII) as previous described[2].Results
An example of the combined skull-plus-brain image is shown in Fig.2. Fig.3 shows the wrap-free images following the proposed unwrapping process that is guided by the low-sensitivity phase data. Fig.4 shows the amplitude and temporal phase delay of the rigid-body translation and rotation of the skull and brain under different vibration patterns. The amplitude of the translation from skull to brain was slightly reduced, whereas the rotation transmission was highly attenuated. The phase delay between the skull and brain rotation was longer (0.80±0.16rad) than that of the translation (0.07±0.01rad). The temporal phase delay was found to be independent of vibrational directions. Fig.5 shows one example of SII to qualitatively visualize the relative skull-brain motion. Note that the skull-brain slip interface is spatially heterogeneous.Discussion
Direct skull displacement measurement is difficult for two reasons: the signal void of the skull on the standard SE-EPI-MRE sequence and heavily wrapped phase in the skull that is topologically separated from the brain. In this study, by switching to water-suppressed acquisition, the medullary cavity filled of fatty marrow has high enough signal to be used to track the skull motion. By using the dual-sensitivity motion encoded acquisition, wrap-free estimates of phase were successfully generated even with only 4 phase offsets, a scenario where conventional phase unwrapping algorithms often fail. We demonstrated that the multi-excitation driver can induce the desired motion patterns. With it, our results suggest that the skull-brain interface may significantly attenuate and delay the rotational motion that is potentially more harmful in regard to brain injury[3]. Interestingly, the slip interface between the skull and brain does not appear to be completely evident. This suggests that some areas of the interface may be mechanically different. Of note, there appears to exist a complete slip interface between the scalp and skull, suggesting that the scalp motion may not be an accurate surrogate measure of skull motion.Conclusion
Our results suggest that the proposed dual-saturation imaging and dual-sensitivity motion encoding MRE technique is an effective tool for measuring 3D in vivo skull and brain motion under different vibrational conditions.Acknowledgements
This work was supported by grant from the National Institute of Health RO1 EB001981.References
[1] Badachhape, A. A. et al. The Relationship of Three-Dimensional Human Skull Motion to Brain Tissue Deformation in Magnetic Resonance Elastography Studies. J Biomech Eng 139, doi:10.1115/1.4036146 (2017). [2] Yin, Z. et al. Slip Interface Imaging Predicts Tumor-Brain Adhesion in Vestibular Schwannomas. Radiology 277, 507-517, doi:10.1148/radiol.2015151075 (2015). [3] Rowson, S. et al. Biomechanical Perspectives on Concussion in Sport. Sports medicine and arthroscopy review 24, 100-107, doi:10.1097/jsa.0000000000000121 (2016).Figures
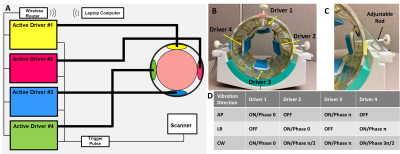
Fig.1. (A)The schematic
diagram and (B)picture of a multi-excitation
MRE head driver. Four soft, pillow-like head drivers were orthogonally positioned
inside of a custom-designed 12-channel head coil and were driven by 4 independent
pneumatic active drivers, respectively. The position of each driver is
adjustable by (C)a plastic rod
attached to it to ensure the optimal contact between the head and the driver.
Memory foam pads were placed between the driver and the head for comfort and to
ensure contact. (D)Three distinct
excitation patterns (anterior-posterior(AP), left-right(LR), and clockwise(CW))
were performed by independently setting the amplitude and phase of the
individual actuators.
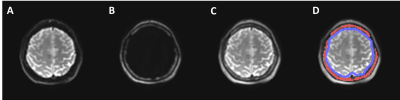
Fig.2. Illustration
of dual-saturation imaging of a healthy volunteer in a slice with large skull
volume. (A) MRE magnitude brain
image acquired with the water-selective spatial-spectral (SPSP) excitation. (B) MRE magnitude image of scalp-skull
acquired with the fat-selective SPSP excitation. (C) MRE magnitude image generated by combining (A) and (B). The
recombined image demonstrates excellent depiction of both the skull and brain. (D) Illustration of the skull (red) and
brain surface (blue) ROIs for motion analysis.
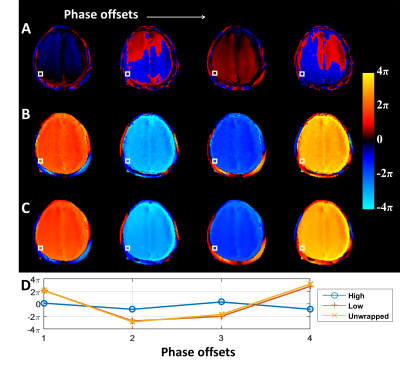
Fig.3. Phase
unwrapping of a volunteer MRE phase data encoded in the AP direction and with
AP vibration. The AP vibration leads to heavily wrapped phase from motion
encoded in the AP direction for both the skull and brain. (A)
Wrapped phase from high-sensitivity encoding, (B) estimated phase from low-sensitivity encoding, and (C) unwrapped phase at 4 MRE phase
offsets (columns). (D) The plot of
the phase values at a single skull voxel (the white boxes) versus the 4 phase
offsets. It correctly showed one period of the sampled sinusoidal wave on low
sensitivity phase and unwrapped phase images.
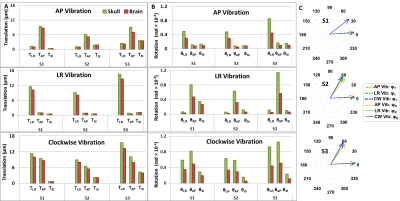
Fig.4. The
amplitude of the rigid-body (A)translation
and (B)rotation components of the
skull and the brain under different vibrations for three volunteers. For both
skull and brain, the largest translation and rotation under AP-vibration were
measured along AP and LR directions, respectively, whereas the largest translation
and rotation under LR-vibration were measured along LR and AP directions,
respectively. Clockwise-excitation leads to large amplitude in both AP and LR
translations and rotations. (C)The
temporal phase delays (Translation:φT and
Rotation:φR)
between the skull and brain motion for each volunteer. Note that in S1, the three φR vectors under 3 vibrations are overlapped.
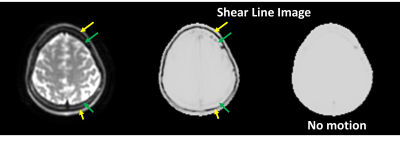
Fig.5. Slip
interface imaging (SII) results of a single volunteer. Clear dark lines were
observed along the scalp-skull interface (yellow arrows), indicating a complete
slip interface between the scalp and the skull. In contrast, fainter dark lines
were only partly observed along the skull-brain interface, indicating less
differential motion between the skull and nearby brain compared to the
scalp-skull interface.