1071
Hemispheric specialisation of hippocampal viscoelasticity for memory performance in healthy older adults1Alzheimer Scotland Dementia Research Centre, University of Edinburgh, Edinburgh, United Kingdom, 2Edinburgh Imaging Facility, QMRI, University of Edinburgh, Edinburgh, United Kingdom, 3Department of Biomedical Engineering, University of Delaware, Newark, DE, United States, 4Thayer School of Engineering, Dartmouth College, Hanover, NH, United States, 5Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana-Champaign, IL, United States
Synopsis
MR Elastography of the hippocampus has been associated with memory performance in young adults and thus may have potential as a novel imaging biomarker for Alzheimer’s disease (AD). In healthy older-adults, hippocampal damping ratio ξ was significantly associated with performance on a verbal memory task. Due to greater hippocampal atrophy present in older populations, the contributions of voxels containing CSF were analysed. Stronger correlations with memory were found once CSF voxels were excluded, and when the left hippocampus was analysed separately. MRE of the hippocampus may be a sensitive marker for detecting early pathological changes in patients with AD.
Introduction
Previous work has found a direct relationship between the viscoelastic properties of brain tissue and cognitive function. Microstructural integrity of hippocampal tissue (i.e. damping ratio ξ), assessed using MR elastography (MRE), has been associated with relational memory performance in healthy young adults1,2. Relationships between brain viscoelasticity and cognition in healthy older adults, however, have not yet been investigated, despite the functional decline experienced by the elderly. Disruptions to episodic memory, which allows an individual to consciously retrieve a previously experienced episode of life, are among the earliest signs and symptoms of AD3. An imaging biomarker related to episodic memory performance is therefore a promising avenue for early AD detection. Previously, the hippocampal MRE approach sought to minimise the corrupting influence of CSF4, but in older subjects a more rigorous exclusion of CSF may be necessary. Furthermore, unilateral hippocampal measures have not been previously studied despite a wealth of literature suggesting that the left hippocampus is involved in verbal/narrative memory, whereas the right has been implicated in visuospatial attention.
Aims
To investigate the possible relationship between hippocampal viscoelasticity and episodic memory performance in cognitively healthy older adults. Two additional aims were to assess the impact of conservative exclusion of CSF-containing voxels, and whether unilateral measurements of the left and right hippocampi are more strongly related to memory performance.Methods
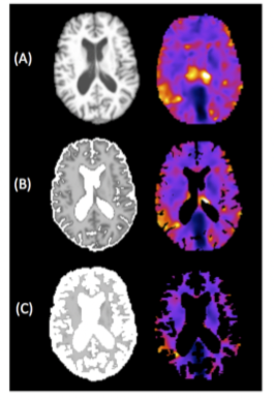
Eleven right-handed participants (mean age=69.1+2.3 years, 6F/5M) were recruited from the Join Dementia Research database. All were required to score >26/30 on the Montreal Cognitive Assessment (MoCA) and performed the National Adult Reading Test (NART) to measure full scale IQ. MRE data were acquired at 50Hz using a multi-shot multi-slab spiral sequence5 and inverted with a FEM-based non-linear inversion (NLI) algorithm incorporating soft prior regularization (SPR)6. MRE data quality was measured by OSS-SNR7. Masks of the hippocampus, and caudate, a control brain region, were generated with Freesurfer and co-registered to MRE maps using FLIRT within FSL. Outcome MRE measures were shear stiffness, μ (kPa) and damping ratio ξ. CSF maps were extracted from a T1-weighted image using FAST within FSL8 and coregistered to the MRE data, with the partial volume map containing intensity values representing the proportion of CSF within each voxel (from 0-100%). Damping ratio ξ images with varying degrees of CSF are illustrated in Figure 1. Episodic memory was assessed by using the Verbal Paired Associates subtest (VPA) from the Wechsler Memory Scale-III; the maximum score for immediate recall was 24.Results
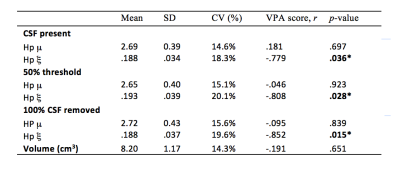
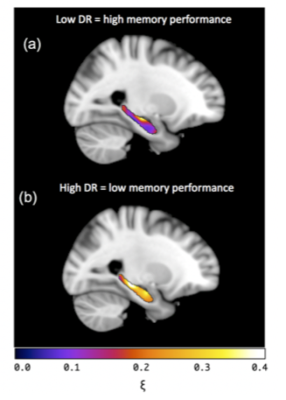
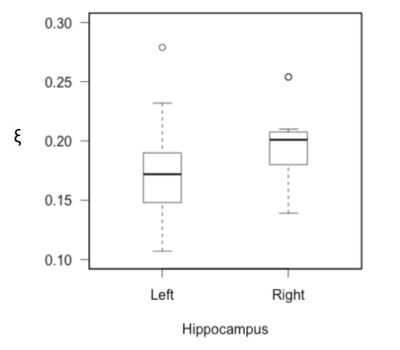
Pearson partial correlation coefficients, r, with age, sex, NART IQ, and ROI volume were used as control variables to investigate how each MRE measure correlated with VPA performance. Results are provided for a) the inclusion of all CSF voxels, b) the exclusion of voxels containing >50% CSF, and c) the removal of all CSF-containing voxels, see Table 1. The significance of correlations was determined at p<0.05. No significant correlation with VPA performance was found for either the global cerebrum or the caudate, including both MRE measures (μ, ξ) and MRI volumetry. No significant correlation with memory performance was found for hippocampal μ, or hippocampal volume. However, hippocampal ξ was negatively correlated with VPA score (r = -.779, p = .036). Stronger correlations were found when voxels containing majority CSF (>50%) were excluded (r = -.808, p = .028), and when CSF voxels were removed entirely (r = -.852, p = .015). Finally, unilateral analysis showed that the left (r = -.928, p = .003), yielded a significantly stronger correlation than the right (r = -.652, p = .112) hippocampus with VPA score (z = 1.66, p < .05), despite no significant difference in hippocampal ξ between hemispheres (t = -1.75, p = .112). Example images of the left hippocampus for a high and low performing subject are provided in Figure 2.
Conclusions
Increase in the relative proportion of viscous-to-elastic behaviour (i.e. higher damping ratio ξ) of the hippocampus is associated with poorer performance on a measure of episodic memory, consistent with previous studies of relational memory. Hippocampal volume, however, was not associated with memory performance despite the well-established relationship with cognitive decline in ageing9. Increase in ξ potentially reflects excessive accumulation of damaged and disorganised tissue components10 that more effectively absorb strain energy. Removal of CSF voxels produced a stronger correlation between ξ and VPA score, suggesting that CSF-containing voxels may adversely affect measurements due to data-model mismatch. Finally, the left hippocampus revealed a significantly stronger relationship to VPA score compared to the right, which may be related to the undoubted role of the left hippocampus in the storage of verbal material. Future work may aim to assess whether the right hippocampus is more strongly related to spatial and pictorial material.Acknowledgements
No acknowledgement found.References
[1] Schwarb H, Johnson CL, McGarry MDJ, et al. Medial temporal lobe viscoelasticity and relational memory performance. NeuroImage. 2016;132(3): 534-41.
[2] Schwarb H, Johnson CL, Daugherty AM, et al. Aerobic fitness, hippocampal viscoelasticity, and relational memory performance. NeuroImage. 2017;153:179:88.
[3] Gold CA, Budson AE. Memory loss in Alzheimer's disease: implications for development of therapeutics. Expert Rev. Neurother. 2008;8(12): 1879-91.
[4] Johnson CL, Schwarb H, McGarry MDJ, et al. Viscoelasticity of subcortical gray matter structures. Hum Brain Mapp, 2016.
[5] Johnson CL, Holtrop JL, McGarry MDJ, et al. 3D multislab, multishot acquisition for fast, whole-brain MR elastography with high signal-to-noise efficiency. Magn Reson Med. 2014;71: 477–485.
[6] McGarry MDJ, Johnson CL, Sutton BP, et al. Including spatial information in nonlinear inversion MR elastography using soft prior regularization. IEEE Trans Med Imaging. 2013;32: 1901–1909.
[7] McGarry MDJ, Van Houten EEW, Perrinez PR, et al. An octahedral shear strain-based measure of SNR for 3D MR elastography. Phys Med Biol. 2011;56: N153–64.
[8] Zhang Y, Brady M, and Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imag. 2001;20(1): 45-57.
[9] Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerb Cortex. 2005;15(11): 1676-89.
[10] Mattson MP, Magnus T. Aging and neuronal vulnerability. Nature Rev Neurosci. 2006;7(4): 278– 294.
Figures