1070
Multi-Excitation MRE in Aging Human BrainAaron T Anderson1,2, Curtis L Johnson3, Tracey M Wszalek2, Bradley P Sutton2,4, Elijah EW Van Houten5, and John G Georgiadis6
1Mechanical Science & Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 2Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 3Biomedical Engineering, University of Delaware, Newark, DE, United States, 4Bioengineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 5Département de génie mécanique, Université de Sherbrooke, Sherbrooke, QC, Canada, 6Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States
Synopsis
The adult aging process affects human brains in different ways and becomes more prone to neurodegenerative diseases. MRE has shown it’s sensitivity to both changes within healthy brains and identifying biomarkers in diseased brains. This study builds on previous MRE aging research and adds higher-resolution, full-coverage MRE imaging and the ability to identify tissue anisotropy, or lack thereof, with the multi-excitation experiment. We were able to identify important anisotropic differences in the loss modulus for some white matter (WM) regions within the young group and a loss of group-level anisotropy in the select WM regions in the older group.
Introduction
Previous studies using MRE have shown the mechanical properties of the healthy aging human brain change with age1-3, but not all areas by the same amount4. DTI literature has shown diffusion anisotropy measures tend to peak around the age of 30-35 years old and decrease with age5. This study is unique in both higher resolution, full brain MRE imaging, necessary for regional comparisons, and capturing anisotropy changes, expected from DTI, using an isotropic material estimation using the multi-excitation experiment6.Methods
The experiment from a previous study [6] was repeated on eight young (AP & LR = 7; 24-32 years old) and seven older (AP & LR = 4; 55-75 years old) males. The MRE displacement imaging employed a 3D multislab, multishot spiral MRE sequence for generating 3D, full vector field complex displacement data at 50 Hz with 2 x 2 x 2 mm3 isotropic spatial resolution7. Multi-excitation experiment includes anterior-posterior (AP) excitation MRE (applied at posterior) and left-right (LR) excitation MRE (applied on right side). The material property estimation was performed using nonlinear inversion (NLI) estimated heterogeneous, isotropic viscoelastic properties8. Additionally, matched field-of-view and resolution (relative to MRE) diffusion tensor imaging (DTI) and T1-weighted MPRAGE structural image at 0.9 x 0.9 x 0.9 mm3 isotropic resolution (TR/TI/TE = 2000/900/2.2 ms) were acquired. MPRAGE is used to register the subjects to atlases, like the MNI JHU white matter (WM) atlas9, for regional-level analysisResults & Discussion
Age is expected to modulate the brain, globally and within WM and GM regions, with a decrease in storage modulus (G') and increase in loss modulus (G''), see example elastograms in Figure 1. For standard AP-excitation NLI estimations, G’ shows an overall decrease with age for the whole brain and the selected white matter (WM) regions, see Figure 2 & 3. G'' shows little change with age for whole brain, corpus callosum (CC), and corona radiata (CR) but increases for superior longitudinal fasciculus (SLF), see Figure 2 & 3. The average change in properties with age for both MRE and DTI are summarized in the table in Figure 2. The rates of decrease are lower for whole brain than previously reported (Sack1 = 7.5 Pa/year and Arani4 = -11 Pa/year), but it is likely caused by the split in a “stiff” and “soft” group within the young subjects, see Figure 3. The only study reporting G'' values show a decrease with age2. The fractional anisotropy (FA) and radial diffusivity (RD) from DTI for a small sample size is reasonably consistent with DTI aging literature6, table shown in Figure 2. The question proposed in the first multi-excitation experiment6 and this study is “how does the wave propagation direction influence the isotropic material estimations?”. The added benefit from the previous study was multi-excitation’s capability to reveal excitation-dependent properties in certain WM regions, which presumably reflect material anisotropy. The difference in material property estimates from the AP and LR estimations was illuminated by the voxel-wise analysis of the average strain asymmetry, defined as $$ \varepsilon_{\parallel/\bot} = \frac{\frac{1}{2}(\varepsilon^f_{12} + \varepsilon^f_{13})}{\varepsilon^f_{23}}$$ where the strains are in the DTI-based fiber reference frame6. Figure 4 shows the difference in properties from AP and LR, with G'' in CC and CR being significantly different in the young group (t-test). Figure 5 takes the values from Figure 4 and computes ratios, within a subject, to highlight how excitation-dependent the properties are for each subject. There are positive correlations for CC and CR, while the SLF has a negative correlation. Surprisingly, CC did not have strain ratios above unity and, for the properties, G' has only a few above unity and G'' has none; thus, the AP and LR excitations behavior very differently in CC.Conclusions
The previous single subject multi-excitation experiment was successfully implemented in a larger cohort and at least part of the results were replicated in the young group (i.e. G'' differences in CC and CR). Utilizing the DTI structural information outside of the heterogeneous, isotropic inversion allowed for capturing anisotropy in the young group and decrease in anisotropy in the older group. For standard MRE, there was a general decrease in G' with age and an increased G'' for the SLF. Given the unique structure of each subject, the group-level excitation-dependence was not strongly apparent; however, the asymmetries within each subject provided insight into individual subject differences. Ultimately, this study furthers two important areas underlying all of brain MRE: characterizing the underlying changes with age and the importance of accurately capturing the anisotropic behavior of certain WM regions.Acknowledgements
Support for ATA and JGG was provided by NSF Grant CMMI-1437113. Partial support was provided by the Biomedical Imaging Center of the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign (UIUC), NIH Grant R01-EB018230, and NIH/NIBIB Grant R01-EB001981. This research is part of the Blue Waters sustained-petascale computing project, which is supported by the National Science Foundation (awards OCI-0725070 and ACI-1238993) and the state of Illinois. Blue Waters is a joint effort of the University of Illinois at Urbana-Champaign and its National Center for Supercomputing Applications.References
[1] Sack, I., Beierbach, B., et al. The impact of aging and gender on brain viscoelasticity. NeuroImage. 2009;46, 652–657. [2] Sack, I., Streitberger, et al. The Influence of Physiological Aging and Atrophy on Brain Viscoelastic Properties in Humans. PLoS ONE. 2011;6, e23451. [3] Guo, J., Hirsch, S., et al. Towards an Elastographic Atlas of Brain Anatomy. PLoS ONE. 2013;8, e71807. [4] Arani, A., Murphy, et al. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. NeuroImage. 2015;111, 59–64. [5] Davis, S.W., Dennis, et al. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. NeuroImage. 2009; 46, 530–541. [6] Anderson, A.T., Van Houten, E.E.W., et al. Observation of direction-dependent mechanical properties in the human brain with multi-excitation MR elastography. JMBBM. 2016;59, 538–546. [7] Johnson, C.L., Holtrop, J.L., et al. 3D multislab, multishot acquisition for fast, whole-brain MR elastography with high signal-to-noise efficiency. Magn. Reson. Med. 2014; 71, 477–485. [8] Van Houten, E.E.W., Paulsen, et al. An overlapping subzone technique for MR-based elastic property reconstruction. Magn. Reson. Med. 1999; 42, 779–786. [9] Hua, K., Zhang, J., et al., Tract probability maps in stereotaxic spaces: analysis of white matter anatomy and tract-specific quantification. NeuroImage. 2008;39(1):336-347.Figures
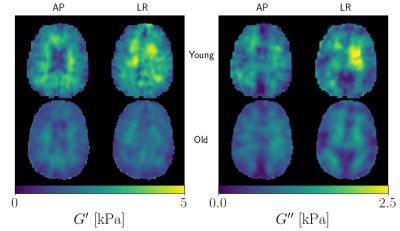
Figure 1: Example material property reconstructions of a
young and old subject for both AP and LR excitations. It is easy to see the
young subject, overall, has higher G’ and G’’, and qualitatively, the AP and LR maps are most
different at the interior of the brain.
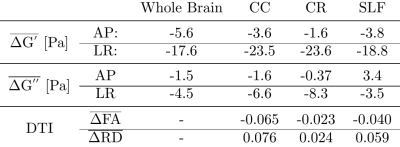
Figure 2: Summary of the average changes with age for G',
G'', and DTI metrics. All values are average relative changes per year. For
both G'
and G'',
the LR excitation appears to be more sensitive to changes with age. Fractional
anisotropy (FA): dimensionless and radial diffusivity (RD): [10-3 mm2/s];
FA and RD compare well with Davis et al.5: $$$\Delta \mathrm{FA}_{CC} \approx
-(0.04-0.05)$$$ and $$$\Delta \mathrm{RD}_{CC} \approx 0.05$$$ [10-3 mm2/s].
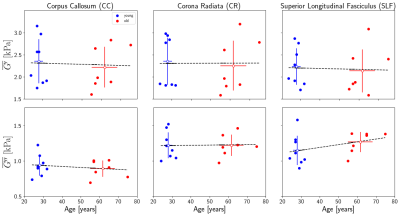
Figure 3: Regional white matter (WM) averages for the
standard AP excitation experiment. There seems to be more subject variation,
young and old, for G’
than G’’
for all of the regions. G’
decreases with age for all three regions, but it decreases slower than other
MRE aging studies. G’’
shows nearly no change with age in CC and CR but increases for SLF. G’’ is
a less reported value and only one study shows a whole-brain decrease with age2.
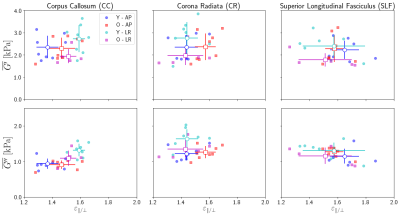
Figure 4: Comparison of the material property estimates
from AP and LR excitation experiments relative to the voxel-wise strain asymmetry.
The loss modulus (G'') in the CC and CR were significantly different in the
young group; however, the older group did not have any significantly
group-level differences. The difference G'' in CR was not associated with a difference in the strain ratio. In the previous study6, there were significant differences
in G'
& G''
for CC and G''
for CR.
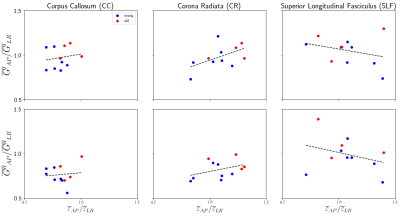
Figure 5: The ratio of material properties from the two
multi-excitation experiments relative to the ratio of strain asymmetry, both AP
to LR, for three WM regions. For strain, the ratios show the relative average asymmetry
experienced in an area for each subject. The G ratio indicates the isotropic
NLI’s discrepancy
in estimating the anisotropic material structure of white matter. CC has a limited strain ratio range (keeping values in the lower-left quadrant), CR experiences a span in strain and thus estimated G, and SLF has large variation (i.e. less organized) in both strain and G.