1069
Assessing tumor mechanical properties and blood perfusion with MRI and correlation with tumor pressure at different compression levels in mice1Laboratory of Imaging Biomarkers, UMR1149, INSERM-University Paris Diderot, Paris, France, 2Sanofi Aventis, Vitry-Sur-Seine, France, 3Department of Radiology, Beaujon University Hospital Paris Nord, Clichy, France
Synopsis
The purpose of this study was to evaluate the changes of total tumor pressure and mechanical properties as a function of increasing stress. MR elastography and perfusion measurements (FAIR method) were performed in mice with tumors xenografted subcutaneously. Tumor pressure was measured with a catheter-transducer system. Measurements were performed at different stress levels by externally compressing the tumor with an inflatable balloon. The results show that increasing the externally applied compression results in increased mechanical properties and tumor pressure and decreased perfusion. These results suggest that elevated tumor pressure can be explained by solid stress rather than fluid pressure.
Introduction
In tumors, high pressure is caused by elevated interstitial pressure and by solid stress related to cancer cell proliferation and desmoplastic reaction. High tumor pressure prevents high molecular weight drugs from being transported to sites of interest, hence methods able to assess tumor pressure in-vivo in growing tumors are potentially very useful. The mechanical properties of tissues are influenced by their solid and liquid components1. The relative influence of the solid stress and fluid pressure remains debated in malignant tumors2-3. Although interstitial fluid pressure can theoretically increase when a tissue is under compression4, the relation with mechanical properties measured with clinically applicable MR elastography (MRE) requires further clarification. This study proposed to alter the relative influences of solid and liquid components in an in-vivo xenograft tumor model, by externally applying a stress, and assess at MRI the changes in mechanical properties and perfusion. The reference examination consisted in local pressure measurements with a miniaturized transducer.Methods
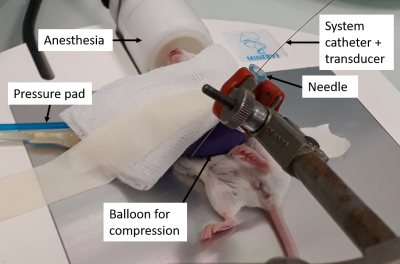
MRI examinations were performed in 11 SCID mice with subcutaneous tumors (patient derived hepatocellular carcinoma xenografts) implanted in the left flank. A 7T MRI scanner (Pharmascan, Bruker, Germany) with a volume resonator and a 25 mm diameter receiver coil was used. Mechanical vibrations were generated with a uniaxial acoustic shaker and transmitted to the tumor via a rigid carbon fiber rod linked to a 3D-printed plastic insert5. An inflatable balloon was placed on the tumor to apply a controlled mechanical stress. MRE and MR FAIR perfusion acquisitions (Figure 1) were performed at basal stress and at increasing stress levels. A sensor placed in the balloon chamber allowed to measure the exerted pressures. The sensor was connected to a pressure transducer (MPXV7007GP, NXP, Eindhoven, The Netherlands) interfaced to an Arduino system (Arduino.cc, 2015). Modulus pseudo-static stress strain ratio was calculated from the slope of the pressure as a function of the strain, measured from MR anatomical images. Mechanical vibrations were performed at 600 Hz and synchronized with a sinusoidal motion-encoded spin echo sequence. MRE acquisitions were performed for each three spatial directions, including 4 times steps. Maps of G’ and G’’ were obtained by inversion of the Helmoltz wave equation6. After MR acquisition, the tumor pressure was measured at different stress levels (Figure 2) using a catheter-mounted piezoelectric pressure transducer (SPR-1000 Mikro-Tip, Millar Instruments, Houston, TX, USA) in five mice.Results
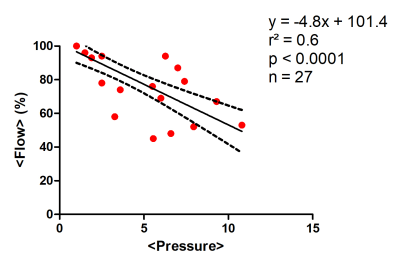
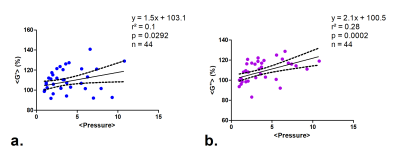
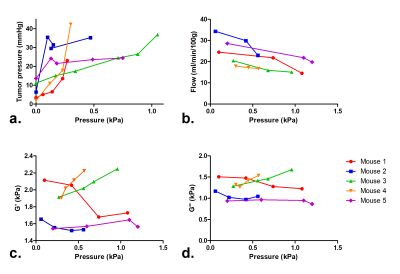
The pseudo-static stress-strain ratio was measured at 3.27 ± 1.79 kPa (n = 11). Figure 3 shows a decrease of tumor perfusion at increasing stress (expressed as the applied balloon pressure). Complex shear modulus measured with MRE (G’ and G’’) is correlated with the applied stress in figure 4. Both G’ and G’’ change as a function of applied pressure with a similar slope. The evolutions of perfusion, mechanical properties and tumor pressure are shown in Figure 5. While the perfusion decreases in all mice, mechanical properties and tumor pressure measurements vary on an individual basis. The behaviors are similar for G’ and tumor pressure. In mice 2 and 5, the mechanical properties and tumor pressure are insensitive to externally applied stress, while in mice 1, 3 and 4, application of external stress results in increased tumor pressure and mechanical properties. Also, mice with the lowest G’ and G’’ have the highest initial sensitivity to applied stress, whereas stiffer tumors respond more progressively.Discussion
In tumors with pseudo-static stress strain ratios similar to those found in a previous study7, our results show that with increasing compression, the tumor elasticity changes according to the applied pressure. This evolution has been previously shown ex-vivo in the liver8 and in tumors9. The mechanical properties and total tumor pressure tend to increase despite the gradual loss of the perfusion component, suggesting that intravascular and free fluid phase pressures are not major determinants of tumor pressure10. The two mice, in which saturable tumor pressure was observed, corresponded to the least stiff but most perfused tumors. These results suggest that in softer tissue the effect of fluid pressure is more determinant.Conclusion
This study shows that with an externally applied tumor stress, there is a tendency to an increase of mechanical properties and tumor pressure, and a decrease of the perfusion component. This suggests that in stiff tumors, intra-tumor pressure is generated predominantly by solid stress and elevated pressure within the interstitial hyaluroninan-rich gel-fluid phase, whereas in softer tumors mechanical properties are more influenced by their liquid component.Acknowledgements
No acknowledgement found.References
1. Sinkus R, et al. MR elastography of breast lesions: understanding the solid/liquid duality can improve the specificity of contrast‐enhanced MR mammography. Magnetic Resonance in Medicine 58.6 (2007): 1135-1144.
2. Chauhan V P, et al. Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer cell 26.1 (2014): 14.
3. DelGiorno K E, et al. Interstitial pressure and vascular collapse in pancreas cancer: fluids and solids, measurement and meaning. Cancer Cell 26.1 (2014): 16.
4. Darling A L, et al. Interstitial fluid pressure in soft tissue as a result of an externally applied contact pressure. Physics in medicine and biology 52.14 (2007): 4121.
5. Ronot M, et al. Viscoelastic parameters for quantifying liver fibrosis: three-dimensional multifrequency MR elastography study on thin liver rat slices. PloS one 9.4 (2014): e94679.
6. Sinkus R, et al. Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magnetic resonance imaging 23.2 (2005): 159-165.
7. Yeh, W Elastic modulus measurements of human liver and correlation with pathology. Ultrasound in medicine & biology 28.4 (2002): 467-474.
8. Clarke E C, et al. Using static preload with magnetic resonance elastography to estimate large strain viscoelastic properties of bovine liver. Journal of biomechanics 44.13 (2011): 2461-2465.
9. Tardieu M, et al. Abstract 4256: Assessing tumor mechanical nonlinearity by MR elastography at different strain levels. In: International Society of Magnetic Resonance in Medicine (2017).
10. DuFort C C, et al. Interstitial pressure in pancreatic ductal adenocarcinoma is dominated by a gel-fluid phase. Biophysical journal 110.9 (2016): 2106-2119.
Figures