1067
Altered brain tissue stiffness in pediatric cerebral palsy measured with magnetic resonance elastography1Biomedical Engineering, University of Delaware, Newark, DE, United States, 2Psychological and Brain Studies, University of Delaware, Newark, DE, United States, 3Orthopedics, Nemours Alfred I. duPont Hospital for Children, Wilmington, DE, United States, 4Department of Kinesiology and Applied Physiology, University of Delaware, Newark, DE, United States
Synopsis
Magnetic resonance elastography (MRE) measures the viscoelastic mechanical properties of tissues, which vary extensively between normal and disease states. In this study, we hypothesized that the mechanical integrity of brain tissue is reduced in children with cerebral palsy (CP). Through MRE, we found the stiffness of the cerebrum in children with CP ages 5-12 is significantly lower than in typically developing (TD) children. This finding indicates that there is a difference in brain tissue health in children with CP that is quantifiable through stiffness measured with MRE.
Introduction
Cerebral palsy (CP) is a neurodevelopmental disorder that results in functional motor impairment and disability in children1. Characterizing brain health in children with CP with MRI can identify neural damage that affects motor performance2, and provide neural targets for rehabilitation strategies3. Quantitative MRI has been used to probe microstructural tissue integrity in CP4,5, and here we propose to use magnetic resonance elastography (MRE) to measure the mechanical properties of the brain in CP. MRE measures are sensitive to brain tissue integrity, and it has been shown that these properties vary extensively between normal and disease states6,7; though MRE studies on children and pediatric conditions is lacking8. In this study, we use MRE as a technique to sensitively assess neural tissue health in pediatric CP.Methods
Participants: Eight children with CP were recruited through the orthopedic surgery clinic at Nemours/A.I. duPont Hospital for Children, along with three typically-developing children as control participants (N=11 total; 5-12 years old). All CP subjects had a Gross Motor Function Classification System (GMFCS) disability score of 1, representing the lowest level of impairment in CP.
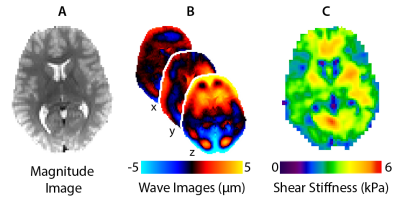
Imaging: MRI scans were completed using a Siemens 3T Prisma scanner and 20-channel head RF-receive coil. MRE data were collected using a single-shot EPI sequence with the following imaging parameters: FOV = 240x240 mm2; matrix = 96x96; 48 slices; TR/TE = 6720/69 ms; GRAPPA R = 3; final resolution = 2.5 x 2.5 x 2.5 mm3. A driver system (Resoundant, Inc.; Rochester, MN) delivered vibrations to the head at 50 Hz. Viscoelastic shear stiffness property maps were generated from MRE displacement images using nonlinear inversion (NLI)9. Figure 1 shows an overview of MRE analyses using a representative TD subject.
Analysis: We quantified the stiffness in the cerebrum for each subject. First, we registered an MNI standard image to MRE space using FLIRT in FSL10,11 followed by an atlas mask of the cerebrum from the WFU PickAtlas12. A one-way ANOVA was used to determine differences between the two groups.
Results
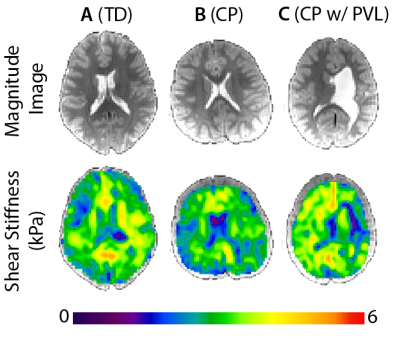
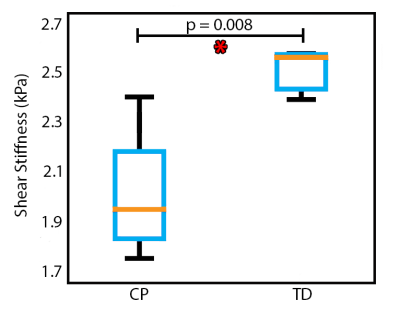
Figure 2 presents example stiffness maps from our population, including a TD child, a child with CP, and a child with CP that exhibited periventricular leukomalacia (PVL), a type of brain damage common in CP. Compared to the TD subject, the CP subject had lower stiffness despite obvious regions of tissue damage from anatomical scans. The CP subject with PVL had an enlarged left ventricle taking over much of the periventricular white matter space; however, the stiffness map did not show evidence of localized damage. The one-way ANOVA shows a significant difference in mean stiffness of the cerebrum between CP (1.99 ± 0.11 kPa) and TD subjects (2.50 ± 0.25 kPa), with p = 0.008 and F = 11.33 (Figure 3). The CP group, on average, had 20.4% lower brain stiffness than the TD group.Discussion
We found that overall stiffness of the cerebrum is significantly lower in CP patients than in TD children representative of a decrease in brain tissue mechanical integrity in children with CP. All CP subjects had a GMFCS disability rating of 1, representing the lowest level of functional motor impairment from the disease. However, we still found a significant difference in shear stiffness between the CP group and the TD group. This preliminary analysis also used a global measure of cerebral stiffness that suggests brain tissue softness from CP is a diffuse phenomenon.
Two
of the eight CP subjects exhibited PVL (Figure 2C), a type of brain damage in
which neuronal cell death occurs in the periventricular white matter and
results in enlarged lateral ventricles, often resulting in spasticity and
intellectual impairment13. Brain tissue softness was not specifically localized to
this region of damage in our participants, but may be in other subjects.
Further analysis will examine stiffness in regions of localized damage, as well
as in white matter and gray matter structures expected to differentially affect
motor and cognitive performance.
Conclusion
This is the first study that has examined the mechanical properties of brain structure in pediatric CP, and our initial results suggest that MRE measures are highly sensitive to brain tissue integrity in this population, even in the absence of severe impairment. These viscoelastic brain properties can be ultimately used to examine relationships in brain health with functional outcome measures, and may elucidate how therapeutic interventions affect neuroplastic recovery in the brains of children with CP.Acknowledgements
This work is supported by the Delaware INBRE program with a grant from the National Institutes of General Medical Sciences (P20-GM103446) from the National Institutes of Health and the State of Delaware.References
1. Bax, M. et al. Proposed definition and classification of cerebral palsy , April 2005 Executive Committee for the Definition of Cerebral Palsy. Dev. Med. Child Neurol. 47, 571–576 (2005).
2. Korzeniewski, S. J., Birbeck, G., DeLano, M. C., Potchen, M. J. & Paneth, N. A Systematic Review of Neuroimaging for Cerebral Palsy. J. Child Neurol. 23, 216–227 (2007).
3. Aisen, M. L. et al. Cerebral palsy: clinical care and neurological rehabilitation. Lancet Neurol. 10, 844–852 (2017).
4. Nagae, L. M. et al. Diffusion Tensor Imaging in Children with Periventricular Leukomalacia: Variability of Injuries to White Matter Tracts. Am. J. Neuroradiol. 28, 1213 LP-1222 (2007).
5. Thomas, B. et al. Quantitative diffusion tensor imaging in cerebral palsy due to periventricular white matter injury. Brain 128, 2562–2577 (2005).
6. Sack, I., Jöhrens, K., Würfel, J. & Braun, J. Structure-sensitive elastography: on the viscoelastic powerlaw behavior of in vivo human tissue in health and disease Emerging Area Soft Matter Background: springpot and viscoelastic scaling. Soft Matter 9, 5672–5680 (2013).
7. Hiscox, L. V et al. Magnetic resonance elastography (MRE) of the human brain: technique, findings and clinical applications. Phys. Med. Biol. 61, R401–R437 (2016).
8. Johnson, C. L. & Telzer, E. H. Magnetic Resonance Elastography for Examining Developmental Changes in the Mechanical Properties of the Brain. Dev. Cogn. Neurosci. (2017). doi:https://doi.org/10.1016/j.dcn.2017.08.010
9. McGarry, M. D. J. et al. Multiresolution MR elastography using nonlinear inversion. Med. Phys. 39, 6388–6396 (2012).
10. Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002).
11. Jenkinson, M. & Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 5, 143–156 (2001).
12. Maldjian, J. A., Laurienti, P. J., Kraft, R. A. & Burdette, J. H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239 (2003).
13. Lee, D. et al. Analysis of structure–function network decoupling in the brain systems of spastic diplegic cerebral palsy. Hum. Brain Mapp. 38, 5292–5306 (2017).
Figures