1054
Myocardial Infarct Border Zone Metabolism Measured by Hyperpolarized 13C-Pyruvate MRI1University of Pennsylvania, Philadelphia, PA, United States, 2Weill Medical Coll of Cornell NY, New York, NY, United States
Synopsis
Adverse remodeling after a myocardial infarct has been linked to the elevated wall stress in the myocardium adjacent to the infarct (i.e. border zone). Perturbed metabolism in this region could drive the transition from compensated to adverse remodeling. To evaluate regional metabolism this study compared the uptake and intracellular conversion of [1-13C]pyruvate using hyperpolarized 13C. An infarct model of ventricular remodeling was used to investigate region metabolism. Pharmacologic stress produced an increase in remote metabolite flux compared to border zone region which may provide a metabolic mechanism for the established association of mechanical stress and adverse cardiac remodeling following infarct.
Introduction:
In spite of significant therapeutic advances, heart failure remains a principal cause of morbidity and mortality with a 5-year mortality rate of 50%.1,2 Myocardial infarct (MI) patients, in particular, are susceptible to the development of heart failure, a debilitating syndrome with poor prognosis. Subsequent to infarction, compensated remodeling of the left ventricular may be followed by adverse remodeling leading to heart failure. The mechanism of adverse remodeling has been linked to the elevated wall stress in the dysfunctional myocardium adjacent to the infarct (i.e. border zone).3 We hypothesize that increased border zone stress results in perturb metabolism in this region which could drive the transition from compensated to adverse remodeling. To evaluate border zone and remote metabolism we compared the regional uptake and intracellular conversion of [1-13C]pyruvate using hyperpolarized (HP) 13C MRS.Methods:
Acute Infarct Model: A well-established pre-clinical posterolateral infarct model of left ventricular remodeling was used to investigate region metabolism. The left anterior descending diagonals and obtuse marginal branches of the left circumflex artery of Yorkshire pigs (n=6) were ligated to create an approximately 20-25% infarct region. This model is characterized by significant left ventricular dilatation with a concomitant decrease in ejection fraction and systolic function, and the development of a border zone region adjacent to the infarct which is normally perfused but hypokinetic. Regional cardiac metabolism was assessed using the MRS protocol, described below, at 6 weeks post-MI. Cardiac function and infarct size were also measured with MRI at the 6-week time-point.
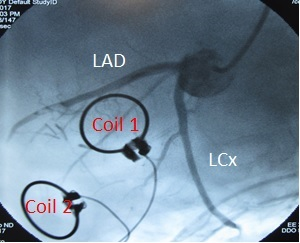
MRS: HP 13C studies were performed on a 3T MR scanner (TIM Trio, Siemens Healthcare) equipped with multi-nuclear capability. Prior to imaging, implantable carbon-tuned receive-only coils were sutured to the LV epicardial surface over the remote and border zone to localize signal to these regions (Figure 1). A catheter was also placed in the left main for direct coronary injection of HP [1-13C]pyruvate to maximize uptake and eliminate signal from the left ventricular cavity. [1-13C]pyruvate was hyperpolarized utilizing the Dynamic Nuclear Polarization method using a HyperSense DNP Polariser (Oxford Instruments). HP solution was prepared with 500 mg of [1-13C]pyruvic acid combined with 1.5mM Gd + 15 mM radical in 7.5 ml H2O + EDTA, melted into 5.5 mL dissolution buffer + 7 mL H2O + EDTA for total volume=20 mL. Solution was injected via the left main catheter over 30 seconds while imaging was performed using a FID sequence with the following parameters: TR/TE=1500ms/0.18ms, FA=25o, Frequency=30.995428 MHz, Measurements=70, Uncombined Coils (Coil1, Coil2), Cardiac Gated. Imaging was performed at baseline (pre-DOB) and during dobutamine (DOB) infusion. Spectra from each coil were analyzed separately and area under the curve (AUC) was measured for each metabolite. Flux was calculated by normalizing metabolite AUC by [1-13C]pyruvate AUC.
Results:
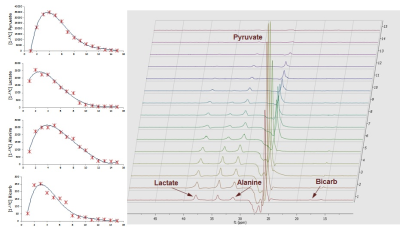
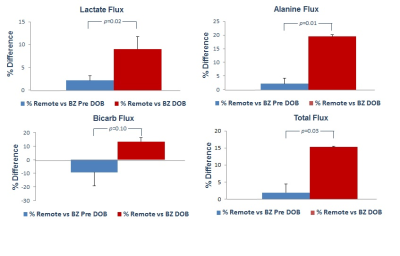
Representative spectra dynamic series and metabolite plots from a single coil acquisition are shown in Figure 2. [1-13C]pyruvate and its metabolites (lactate, alanine, and bicarbonate) are clearly observed at their respective chemical shifts. Under physiologic conditions (Pre-DOB) the percent difference between remote and border zone lactate, alanine, and total flux was only slightly elevated in the remote region whereas border zone bicarb flux was greater compared to remote (Figure 3). DOB infusion significantly altered [1-13C]pyruvate metabolic flux in both the remote and border zone regions. DOB stress produced an increase in remote metabolite flux compared to border zone with lactate, alanine and total flux reaching significance and bicarb flux shifting from greater in border zone Pre-DOB to greater in remote.Discussion:
Using hyperpolarized MRS this study investigated the effect of elevated border zone wall stress on metabolic function. The border zone metabolic profile under normal physiologic conditions was slightly altered compared to the remote region. DOB stress amplified the border zone and remote regional differences by elevating left ventricular workload increasing the metabolic demands for both regions. Border zone flux of [1-13C]pyruvate through pyruvate dehydrogenase (PDH) as assessed by the production of 13C-bicarbonate was 13.6% lower than remote myocardium. In addition, the pyruvate flux through both lactate dehydrogenase (LDH), measured by the production of 13C-lactate, and alanine transaminase (ALT), assessed by 13C-alanine production, were reduced in the border zone compared to remote. Collectively, the lower fluxes observed in the border zone suggest an insufficiency of this region to meet the metabolic demands during periods of increased ventricular workload.Conclusion:
These findings demonstrate an impaired metabolic response to pharmacologic stress in border zone myocardium which may provide a mechanism for the established association of mechanical stress and adverse cardiac remodeling following infarct.Acknowledgements
Study Funding
National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001878.
R01-HL063954 (PI: R. Gorman) Surgery to Prevent Post Infarction Ventricular Remodeling
References
1. American Heart Association Writing Group. Heart Disease and Stroke Statistics—2016 Update: a report from the American Heart Association. Circulation 2016;125:e2– e220.
2. Hellermann JP, Jacobsen SJ, Redfield MM, et al. Heart failure after myocardial infarction: clinical presentation and survival. Eur J Heart Fail 2005;7:119–125.
3. Jackson BM, Gorman III JH, Moainie S, et al. Extension of Borderzone Myocardium in Post infarction Dilated Cardiomyopathy. J Am Coll Cardiol. 2002 Sep 18; 40(6):1160-7.
Figures