1052
Fetal whole-heart 3D cine reconstruction using motion-corrected multi-slice dynamic imaging1School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 2Department of Congenital Heart Disease, Evelina London Children's Hospital, London, United Kingdom, 3Centre for the Developing Brain, King's College London, London, United Kingdom
Synopsis
Cine fetal cardiac imaging with whole heart coverage presents numerous challenges due to maternal and fetal motion as well as high heart rate and lack of reliable independent synchronisation signal. To overcome these challenges, highly accelerated multi-slice 2D dynamic images were retrospectively reordered in time and aligned in space to produce a 3D cine using scattered interpolation methods with outlier rejection. The proposed methods were tested on ten human fetal subjects and validated using a numerical phantom.
Introduction
Fetal cardiac cine MRI has been limited by challenges associated with imaging a small, rapidly beating heart that is subject to various regular and spontaneous movements within the maternal torso1. However, as motion-tolerant methods are developed and improved2,3,4, there is an increasing interest in the use of MRI to visualise fetal cardiac anatomy5,6,7. The successful application of methods for high-resolution 3D depiction of the fetal brain using retrospective motion correction and volumetric reconstruction of two-dimensional MRI8,9 to static MR images of the fetal heart4,10 suggest the potential of this framework for 3D cine cardiac imaging in the presence of motion.
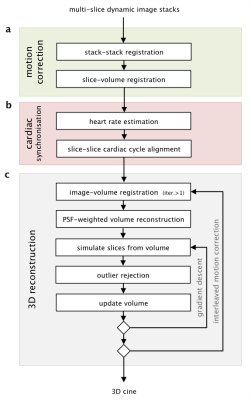
In this work, 3D capability is achieved using highly accelerated 2D dynamic balanced steady state free precession (bSSFP) acquisition combined with retrospective image-domain techniques for motion correction, cardiac synchronisation, and outlier rejection (Fig1).
Methods
Imaging was performed in ten consecutive singleton pregnancies (gestational ages 24-33 weeks, three volunteer, seven cardiac anomaly) on a 1.5T Philips Ingenia when examination time permitted. Scans were performed without maternal breath-hold using anterior torso and posterior spine coil arrays. Stacks of multi-slice dynamic images were acquired in 3+ planes covering the fetal heart using a 2D bSSFP sequence (TR/TE 3.8/1.9ms, flip angle 60°, 8x acceleration, field of view 400×304mm, voxel size 2.0×2.0x6.0mm, slice overlap 3-4mm, 96 images per slice in 7s, 3-6 stacks, 35-75 slices) and reconstructed using k-t SENSE11. Operation was constrained to ensure fetal and maternal safety (less than 2W/kg whole body SAR, 110dB(A) sound pressure level, 60% threshold dB/dt PNS), resulting in reduced scanner performance.
Motion Correction (Fig1a) Spatial alignment using rigid body registration was performed in two stages to promote convergence. The temporal mean (TM) of the dynamic images have less noise and no cardiac pulsation. Stacks of TM images were first aligned using stack to stack registration, and then used to reconstruct a static volume for slice to volume registration. This yielded an initial estimate of the transformation for each slice that were then used in the 3D cine reconstruction. Subsequently, image to volume registration was interleaved with 3D cine reconstruction to improve spatial alignment.
Cardiac Synchronisation (Fig1b) The fetal heart rate for each slice of dynamic images was estimated by identifying the fundamental frequency in the temporal frequency spectrum in a region over the fetal heart, with the estimates of ambiguous spectra (e.g. fetal movement, lack of coverage) replaced with the linear interpolation of neighbouring slices. Synchronisation of the cardiac cycles between slices was determined using cines generated for each slice of dynamic images, using the TM transformations. A cardiac phase offset was applied using a temporal Fourier shift, and slices were synchronised by maximising the Pearson’s correlation between slices.
3D Cine Reconstruction (Fig1c) Methods for static volume reconstruction9 were extended to include a temporal kernel-weighting2 in the image acquisition model. An initial estimate of the 3D cine was generated by inverting the acquisition model, using estimates of the spatial and temporal alignment of each dynamic image frame with the 3D cine, and refined by super-resolution methods9 to recover structures obscured by blurring in the through-slice direction.
Outlier Rejection (Fig1c) Robust statistics were incorporated in the 3D cine reconstruction to exclude inconsistent data so that voxels that were corrupted by motion artifact and frames that were misaligned in space or time were down-weighted in the reconstruction or rejected completely.
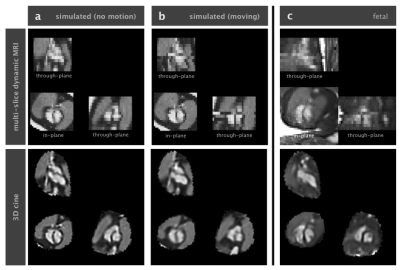
Simulation (Fig2) Methods were validated using images simulated from a numerical phantom12, adapted to the fetus, using the image acquisition model to generate dynamic images with high in-plane resolution and blurring in the through-slice direction. These images were then down-sampled in the in-plane direction by truncating k-space and noise was added. Data was simulated with motion histories taken from fetal cases.
Results
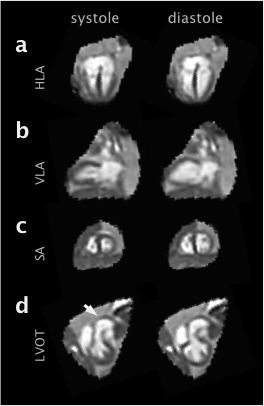
Simulations (Fig2) confirmed anatomical accuracy in the reconstructed 3D cines, though some blurring was evident in the reconstruction of data simulated with motion. The proposed method produced 3D cines (Fig3) that reveal the complex connectivity of the fetal cardiac anatomy (Fig4). Fetal 3D cines were reviewed by a fetal cardiologist (DL) and confirmed to show key features in agreement with ultrasound in eight cases. In two of the fetal cases, large fetal displacements and regular movement lead to poor results. It is expected that manual exclusion of input images may overcome this. The reconstructed 3D cines allow for multiple planes to be visualised (Fig5) with reduced noise and artefact, without requiring precise scan plane prescription during acquisition.Conclusions
The presented method proved robust when used on fetal data and simulations confirmed that correct spatial and temporal features could be reliably recovered. Future work will focus on improving the native spatial resolution, further enhancing reconstructed image quality and initiating clinical studies.Acknowledgements
This work was supported by the Wellcome EPSRC Centre for Medical Engineering at Kings College London [WT 203148/Z/16/Z], the Wellcome Trust iFIND project [102431], MRC strategic grant [MR/K006355/1] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
- Votino C, Jani J, Damry N, Dessy H, Kang X, Cos T, Divano L, Foulon W, De Mey J, Cannie M. Magnetic resonance imaging in the normal fetal heart and in congenital heart disease. Ultrasound Obstet. Gynecol. 2012;39:322–9. doi: 10.1002/uog.10061.
- van Amerom JFP, Lloyd DFA, Price AN, Murgasova MK, Aljabar P, Malik SJ, Lohezic M, Rutherford MA, Pushparajah K, Razavi R, Hajnal JV. Fetal cardiac cine imaging using highly accelerated dynamic MRI with retrospective motion correction and outlier rejection. Magn. Reson. Med. 2017. doi: 10.1002/mrm.26686.
- Roy CW, Seed M, Kingdom JC, Macgowan CK. Motion compensated cine CMR of the fetal heart using radial undersampling and compressed sensing. J. Cardiovasc. Magn. Reson. 2017;19:29. doi: 10.1186/s12968-017-0346-6.
- Lloyd DFA, Kainz B, van Amerom JFP, Pushparajah K, John M. Three-Dimensional Modelling of the Fetal Vasculature from Prenatal MRI using Motion-Corrected Slice-to-Volume Registration. ISMRM 2016. p.1677
- Roy CW, Seed M, Macgowan CK. Preliminary Experience Using Motion-Robust Dynamic MRI to Visualize Fetal Congenital Heart Disease: Comparison to Static MRI. ISMRM 2017. P.4815.
- Yerly J, Chaptinel J, Mivelaz Y, Prsa M, Alamo L, Vial Y, Berchier G, Rohner C, Stuber M. Identification and Measurement of Anatomical Landmarks Using Fetal Cardiac Cine MRI in Comparison with the Clinical Gold Standard Echocardiography. ISMRM 2017. p.3163.
- Lloyd DFA, Amerom JFP Van, Murgasova M, Kainz B, Pushparajah K, Rutherford M, Hajnal J V, Razavi R. Insights from comprehensive fetal cardiovascular MRI assessment using 3D motion-correction and metric-optimised gated phase contrast in cases of suspected coarctation of the aorta. ISMRM 2017. p.3261.
- Jiang S, Member S, Xue H, Glover A, Rutherford M, Rueckert D, Hajnal J V. MRI of moving subjects using multislice Snapshot images with Volume Reconstruction (SVR): Application to fetal, neonatal, and adult brain studies. IEEE Trans. Med. Imaging 2007;26:967–980.
- Kuklisova-Murgasova M, Quaghebeur G, Rutherford MA, Hajnal J V., Schnabel JA. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med. Image Anal. 2012;16:1550–64. doi: 10.1016/j.media.2012.07.004.
- van Amerom JF, Lloyd DA, Murgasova MK, Price AN, Malik SJ, Aljabar P, Allsop JM, Gomes A, Rutherford MA, Pushparajah K, Razavi R, Hajnal JV. Fetal cardiac volume reconstruction from motion-corrected multi-slice dynamic MRI. ISMRM 2017. p.3263.
- Tsao J, Boesiger P, Pruessmann KP. k-t BLAST and k-t SENSE: dynamic MRI with high frame rate exploiting spatiotemporal correlations. Magn. Reson. Med. 2003;50:1031–42. doi: 10.1002/mrm.10611.
- Wissmann L, Santelli C, Segars WP, Kozerke S. MRXCAT: Realistic Numerical Phantoms for Cardiac Magnetic Resonance Imaging. J. Cardiovasc. Magn. Reson. 2014;16:63. doi: 10.1186/s12968-014-0063-3.
Figures