1050
Super Resolution MRI Using 3D Generative Adversarial Network: Towards Single Breath-Hold Coronary MR Angiography1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Ming Hsieh Department of Electrical Engineering, University of Southern California, Los Angeles, CA, United States, 3Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 4Division of Cardiology, Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea
Synopsis
Coronary MRA is an attractive imaging tool to offer noninvasive, radiation-free evaluation of coronary artery disease. However, long scan time and sensitivity to motion limit its current clinical applications. In this paper, we propose a
Introduction
Coronary MR angiography (MRA) is an attractive diagnostic tool for the evaluation of coronary artery disease (CAD) as a noninvasive, radiation-free imaging technique. However, coronary MRA is yet to be used clinically despite over 20 years of continuous technical improvements, as long-lasting limitations such as prolonged scan time and motion sensitivity remain major roadblocks. Recent developments in advanced motion correction1 and high dimensional imaging2 are pushing on these boundaries, however, the additional software and hardware requirements may limit their availability for the vast majority of existing scanners. In computer vision, deep learning approaches have been widely adopted for various tasks such super resolution (SR) and classification.3,4 In this work, we propose a deep learning algorithm based on 3D generative adversarial network (GAN) to recover lost anatomical details in vastly under-sampled coronary MRA data, with the ultimate goal to allow whole-heart coronary MRA in a single breath-hold.Methods
Coronary MRA acquisition: with institutional review board approval and informed consent, we enrolled 49 patients with suspected CAD who had new onset or recurrent stable chest pain, and were scheduled to undergo invasive coronary imaging. Contrast-enhanced coronary MRA were acquired within one week prior to coronary catheterization, on a 3.0T MRI system (MAGNETOM Trio; Siemens Healthineers, Erlangen, Germany) with the following imaging parameters: inversion recovery prepared spoiled gradient echo sequence (IR-FLASH), slow contrast media injection at the dose of 0.20 mmol/kg of Gd-DOTA (Dotarem; Guerbet Group, Villepinte, France) at rate of 0.20 mL/s; inversion time = 250ms; spectral fat saturation; FoV = 218x350x72mm3; slab orientation = transverse; spatial resolution = 1.0x1.0x1.5mm3 (interpolated to 1.0mm isotropic); iPAT = x2 (GRAPPA); bandwidth = 676Hz/pixel; respiratory navigator gated, window width = ±3mm; scan time = 5’55”±1’51”.
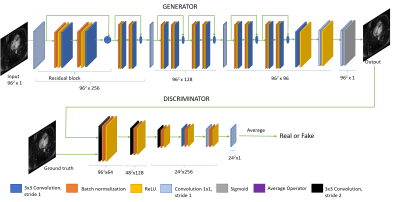
SR reconstruction: as depicted in Figure 1, the purposed super resolution model was designed based on the Generative Adversarial Network5 and implemented using TensorFlow6 in a 2D version and a 3D version. Out of the 49 sets of coronary MRA, 37 were randomly selected as the training set, and the rest 12 were used as the testing set. Under-sampled MRA were generated by truncating the outer 3/4 k-space data in each of the 2 phase encoding directions (head-foot and anterior-posterior). A hamming window was applied to reduce Gibbs ringing. All images were divided into patches of size 96x96 for 2D data or patches of size 48x48x48 for 3D data, respectively. The initial learning rate of generator and discriminator were 10-4 and 10-5 for 2D and 3D, respectively. The learning was decreased to 1/10 of the current learning approximately every three epochs (for 3D data) or 10 epochs (for 2D data). All training was performed with a graphic card (Quadro M6000; NVIDIA, Santa Clara, CA, USA).
Results
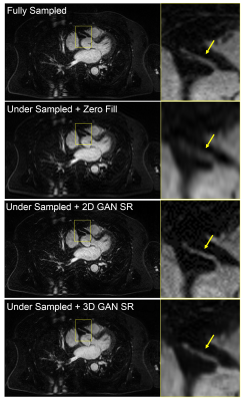
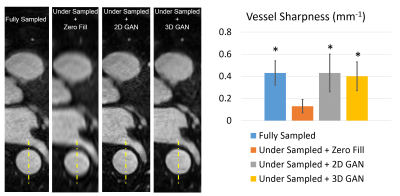
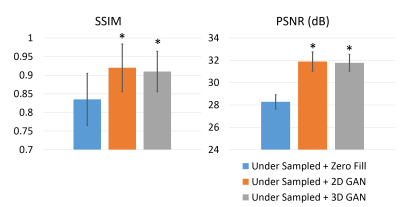
Figure 2 is a representative coronary MRA dataset comparing the visualization of coronary arteries in the testing data between different methods. Figure 3 demonstrates the measurement of vessel sharpness and an overall comparison among different images in the testing set, with the proposed methods showing no significant difference versus the ground truth. Figure 4 shows the quantitative assessment of image quality improvement and detail recovery offered by the proposed method compared with the basic zero-filling approach, using structural similarity index (SSIM) and peak signal-to-noise ratio (PSNR).Discussion
The retrospective under-sampling scheme tested in this experiment consisted of 4x reduction of k-space data in each of the 2 phase encoding directions, an equivalent of 10.7x overall acceleration considering the initial 33% interpolation. Assuming a typical acquisition window of 28 lines per heartbeat, such acceleration factor would reduce the total scan time to 18 heartbeats, which could be finished with a single breath-hold. The perceived gain in image quality and details warrant further validation with prospective under-sampling.
An alternative direction to utilize the proposed super resolution framework would potentially allow overcoming the current spatial resolution barrier in coronary MRA. If an ultra-high resolution coronary MRA dataset can be acquired, possibly through animal experiment under well-controlled condition or ultra-high field MRI, such dataset can be used in the training of 3D GAN and help “upscale” the spatial resolution in the conventional coronary MRA.
Conclusion
The proposed super resolution algorithm based on 3D GAN demonstrated the capacity to improve vessel sharpness and restore fine anatomical details in highly under-sampled coronary MRA data, offering the potential for imaging acceleration and spatial resolution improvement.Acknowledgements
No acknowledgement found.References
1. Pang, J., Chen, Y., Fan, Z., Nguyen, C., Yang, Q., Xie, Y. and Li, D., 2016. High efficiency coronary MR angiography with nonrigid cardiac motion correction. Magnetic resonance in medicine, 76(5), pp.1345-1353.
2. Piccini, D., Feng, L., Bonanno, G., Coppo, S., Yerly, J., Lim, R.P., Schwitter, J., Sodickson, D.K., Otazo, R. and Stuber, M., 2017. Four‐dimensional respiratory motion‐resolved whole heart coronary MR angiography. Magnetic resonance in medicine, 77(4), pp.1473-1484.
3. Dong, C., Loy, C.C., He, K. and Tang, X., 2016. Image super-resolution using deep convolutional networks. IEEE transactions on pattern analysis and machine intelligence, 38(2), pp.295-307.
4. Radford, A., Metz, L. and Chintala, S., 2015. Unsupervised representation learning with deep convolutional generative adversarial networks. arXiv preprint arXiv:1511.06434.
5. Ledig, C., Theis, L., Huszár, F., Caballero, J., Cunningham, A., Acosta, A., Aitken, A., Tejani, A., Totz, J., Wang, Z. and Shi, W., 2016. Photo-realistic single image super-resolution using a generative adversarial network. arXiv preprint arXiv:1609.04802.
6. Abadi, M., 2016, September. Tensorflow: learning functions at scale. In Proceedings of the 21st ACM SIGPLAN International Conference on Functional Programming, pp. 1-1.
Figures