1045
Isotropic resolution DTI of lower back nerves using a phase-corrected diffusion-prepared 3D TSE1Interventional and Diagnostic Radiology, Technical University of Munich, Munich, Germany, 2Institute of Medical Engineering, Technical University of Munich, Garching, Germany, 3Philips Healthcare, Hamburg, Germany, 4Interventional and Diagnostic Neuroradiology, Technical University of Munich, Munich, Germany
Synopsis
Diffusion-prepared 3D TSE (dprep-3D TSE) has been applied for isotropic-resolution distortion-free proximal- and peripheral-nerve DWI. Dprep-3D TSE has been combined with magnitude stabilizers to reduce magnitude modulation induced by motion and eddy currents and has used velocity compensation to reduce motion-induced phase modulation in diffusion-weighted signals. However, due to the multi-shot nature of dprep-3D TSE, remaining motion-induced phase leads to image and DTI-parameter artifacts and requires phase navigation. The purpose of this work is to develop a phase-navigation and phase-correction scheme for dprep-3D TSE and to apply the developed method in vivo for isotropic-resolution DTI of lumbosacral and sciatic nerves.
Purpose
Diffusion-prepared TSE (dprep-TSE) sequences are emerging in diffusion-weighted (DW) proximal- and peripheral-nerve imaging given their ability to achieve higher spatial resolutions without suffering from the geometric distortions characteristic of single-shot echo planar imaging (ss-EPI)1-5. Diffusion-prepared TSE sequences can be combined with 3D readouts to achieve isotropic resolutions, often necessary for imaging nerves in the body6,7. In multi-shot TSE DWI, phase errors induced by motion and eddy currents during diffusion encoding affect the magnitude and phase of the dprep-3D TSE signal8. The use of magnitude stabilizers minimizes magnitude modulation1 while velocity compensation reduces motion-induced phase modulation. It has been shown, however, that the variation of residual motion-induced phase across k-space segments (shots) remains a significant source of artifacts and produces errors in DTI-parameter quantification9. The purpose of this work is to develop a phase-navigated diffusion-prepared 3D TSE acquisition and a modified reconstruction incorporating inter-shot phase-error correction and to show the reduction of phase errors in vivo in the lumbosacral and sciatic nerves.Methods
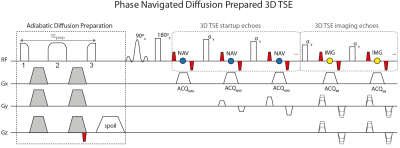
Sequence development: The used diffusion preparation (Fig.1) used a modified BIR-4 RF pulse, shown to increase robustness against B0 and transmit B1 effects in T2 preparation10-13. Diffusion-sensitizing gradients used the Stejskal-Tanner design to minimize the preparation duration. A magnitude-stabilizing gradient was placed before signal tip-up and later balanced in the 3D TSE readout for minimizing phase errors from motion and eddy currents1. The 3D TSE readout acquired imaging echoes using phase encoding in ky and kz. Navigator echoes were acquired, per shot, during the startup-echoes of 3D TSE using phase encoding in ky only.
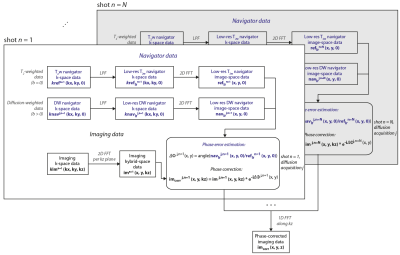
Phase correction and reconstruction: (Fig.2) Low-resolution T2-weighted (T2w) navigator k-space data kreflrn(kx,ky,0) and low-resolution diffusion-weighted (DW) navigator k-space data knavlrj,n(kx,ky,0) were 2D-reconstructed per shot n and diffusion acquisition j. The phase of the resulting jth DW navigator image-space data navlrj,n(x,y,0) was compared, per shot n, to the phase of the T2w navigator image-space data reflrn(x,y,0) (reference). The estimated phase-difference map ΔΦj,n(x,y) was applied on the 2D-reconstructed imaging hybrid-space data imj,n(x,y,kz) to correct for j- and n-dependent phase by means of complex multiplication. 1D reconstruction along kz across all shots resulted in the final phase-corrected imaging data imcorr(x,y,z).
In vivo measurements: DTI of the lumbosacral and sciatic nerves of nine healthy volunteers was conducted with the developed dprep-3D TSE and with ss-EPI on a 3T Philips system using a 16-channel torso coil and the integrated 12-channel posterior coil. DTI with dprep-3D TSE was performed coronally with sequence parameters: FOV=400×400×50mm3, acquisition-voxel=2.5×2.5×2.5mm3, TR/TE=1500/35ms, TSEfactor=53, SENSE-reduction-factor=3, averages=2, b-values=0,400, DTI-directions=6, TEprep=32ms, scan-duration=11m58s. DTI with ss-EPI was performed axially (to minimize distortions) and used as a reference for assessing motion-induced phase effects in dprep-3D TSE. Sequence parameters: FOV=400×250×250mm3, acquisition-voxel=2.5×2.5×2.5mm3, slice-gap=0mm, TR/TE=23832/47ms; averages=3, partial-Fourier-reduction-factor=0.74, SENSE-reduction-factor=2, b-values=0,400, DTI-directions=6, scan-duration=9m8s.
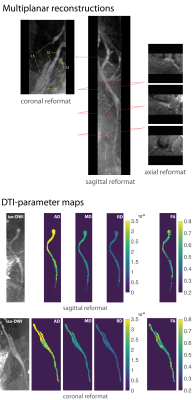
DTI and statistical analysis: AD, RD, MD and FA maps were computed from DTI data acquired with dprep-3D TSE, reconstructed with and without phase correction, and with ss-EPI. ROIs were drawn on the sciatic nerves in iso-DWIs and used to extract DTI-parameter values. Mean DTI-parameter values were compared across subjects using paired t-tests with significance of 5% for (i) dprep-3D TSE with and without phase correction and for (ii) phase-corrected dprep-3D TSE and ss-EPI.
Results
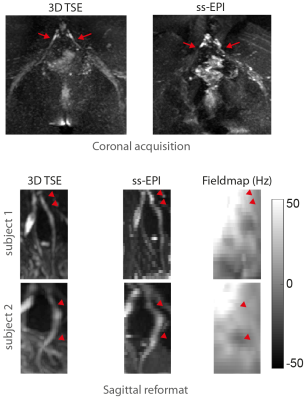
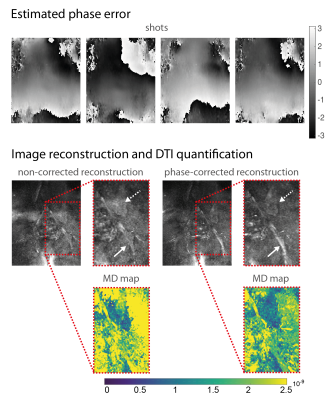
DWI with ss-EPI resulted in significant geometric distortions of lumbosacral nerves compared to dprep-3D TSE (Fig.3). Estimated phase-error maps from dprep-3D TSE show motion-induced inter-shot variations (Fig.4) and corresponding reconstructed DWIs show artifacts from inter-shot phase inconsistencies that are reduced after phase correction. Motion-induced phase errors resulted in large overestimations of MD, which were restored to reasonable values after phase correction. Phase-corrected DTI produced isotropic-resolution iso-DWIs and DTI-parameter maps clearly delineating lumbosacral and sciatic nerves (Fig.5). DTI with phase-corrected dprep-3D TSE resulted in statistically significant reductions in AD, MD and RD in the sciatic nerve compared to non-phase-corrected dprep-3D TSE (P<0.001). DTI with ss-EPI compared to phase-corrected dprep-3D TSE resulted in non-significant differences in AD, MD and FA (P=0.14,P=0.05,P=0.51,resp.).Discussion & Conclusion
The present results confirm that motion-induced phase modulation in dprep-3D TSE varies between shots and leads to considerable artifacts and DTI-parameter errors. Phase correction is shown to minimize artifacts and yield DTI-parameter values consistent with previous reports14,15. Significantly lower sciatic-nerve diffusivity values with phase-corrected dprep-3D TSE compared to non-phase-corrected dprep-3D TSE confirm motion corruption of the uncorrected diffusion signals. Insignificant differences between phase-corrected dprep-3D TSE and ss-EPI DTI-parameter values indicate a comparable accuracy between the two methods. The present work therefore introduces a phase-navigation and phase-correction method for diffusion-prepared 3D TSE at no time penalty that allows distortion-free, isotropic-resolution, 3D DTI of lower back nerves with robustness to motion-induced phase effects.Acknowledgements
The present work was partially supported by Philips Healthcare and received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 637164 — iBack — ERC-2014-STG).References
1. Alsop, D.C., Phase insensitive preparation of single-shot RARE: application to diffusion imaging in humans. Magn Reson Med, 1997. 38(4): p. 527-33.
2. Zhang, Q., et al., Diffusion-prepared stimulated-echo turbo spin echo (DPsti-TSE): An eddy current-insensitive sequence for three-dimensional high-resolution and undistorted diffusion-weighted imaging. NMR in Biomedicine, 2017. 30(7): p. e3719--12.
3. Xie, Y., et al., High resolution 3D diffusion cardiovascular magnetic resonance of carotid vessel wall to detect lipid core without contrast media. J Cardiovasc Magn Reson, 2014. 16: p. 67.
4. Pipe, J.G., Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn Reson Med, 1999. 42(5): p. 963-9.
5. Zhou, X.J., et al., Characterization of benign and metastatic vertebral compression fractures with quantitative diffusion MR imaging. AJNR Am J Neuroradiol, 2002. 23(1): p. 165-70.
6. Chhabra, A., et al., High-resolution 3T MR neurography of the brachial plexus and its branches, with emphasis on 3D imaging. AJNR Am J Neuroradiol, 2013. 34(3): p. 486-97.
7. Kasper, J.M., et al., SHINKEI--a novel 3D isotropic MR neurography technique: technical advantages over 3DIRTSE-based imaging. Eur Radiol, 2015. 25(6): p. 1672-7.
8. Van, A.T., et al., Analysis of phase error effects in multishot diffusion-prepared turbo spin echo imaging. Quantitative Imaging in Medicine and Surgery, 2017. 7(2): p. 238-250.
9. Cervantes, B., et al. One-dimensional phase navigation of diffusion-weighted 3D TSE for high resolution musculoskeletal diffusion imaging. in In: Proceedings of the 25th Annual Meeting of ISMRM. 2017. Honolulu, Hawaii, USA.
10. Cervantes, B., et al. High-Resolution DWI of the Lumbar Plexus using B1-insensitive Velocity-Compensated Diffusion-Prepared 3D TSE. in In: Proceedings of the 24th Annual Meeting of ISMRM. 2016. Singapore, Singapore.
11. Jenista, E.R., et al., Motion and flow insensitive adiabatic T2 -preparation module for cardiac MR imaging at 3 Tesla. Magn Reson Med, 2013. 70(5): p. 1360-8.
12. Nezafat, R., et al., Spectrally selective B1-insensitive T2 magnetization preparation sequence. Magn Reson Med, 2009. 61(6): p. 1326-35.
13. Weidlich, D., et al., T2 mapping with magnetization-prepared 3D TSE based on a modified BIR-4 T2 preparation. NMR Biomed, 2017.
14. Karampinos, D.C., et al., Diffusion tensor imaging and T2 relaxometry of bilateral lumbar nerve roots: feasibility of in-plane imaging. NMR Biomed, 2013. 26(6): p. 630-7.
15. Simon, N.G., et al., Peripheral nerve diffusion tensor imaging is reliable and reproducible. J Magn Reson Imaging, 2016. 43(4): p. 962-9.
Figures