1043
Visualisation and quantification of collagen fibers in a partially torn ligament using magic angle imaging1Medicine, Surgery and Cancer, Imperial College London, London, United Kingdom, 2University of Adelaide, Adelaide, Australia, 3Mechanical Engineering, Imperial College London, London, United Kingdom, 4Department of Clinical Sciences and Services, Royal Veterinary College, Hertfordshire, United Kingdom
Synopsis
Human partial anterior cruciate ligament tears can be extremely difficult to diagnose with conventional MRI. Variations of signal intensity within the ligament are suggestive of injury but it is not possible to confirm damage or assess the collagen alignment within the ligaments. We have shown that magic angle imaging has the ability to visualise and quantify collagen fibers in a partially torn canine cruciate ligament. Furthermore it can delineate between damaged and healthy fiber bundles within the same ligament. This method has the potential to become a non-invasive alternative to arthroscopy for assessing and monitoring ligament damage and repair outcomes.
Introduction
The potential use of magic angle imaging as a collagen contrast mechanism is not a new idea, however harnessing its potential has remained a challenge1. It has been suggested that ligament tearing or significant joint degeneration would decrease tissue anisotropy and therefore reduce the magic angle effect2. Bovine tendons which were damaged post mortem (PM) with collagenase and glucose demonstrated a reduction in the magic angle effect3. Horse tendons damaged PM by laser diode no longer demonstrated a magic angle effect4. Spontaneous cruciate ligament disease and rupture is relatively common in dogs. Ten canine knees from animals euthanized for clinical reasons unrelated to the study were imaged, then dissected to compare imaging with veterinary diagnosis.Methods
Ethical approval was granted by the Royal Veterinary College London (URN 2017 1659-3) to collect knees from dogs who had been euthanized and required a post mortem. The canine knees were scanned on a Siemens Verio 3T using a 12 channel head coil fitted with a specially designed holder and test sphere containing the embedded knees. The sphere was scanned in 9 directions to the main magnetic field (B0). An isotropic 3D T1 FLASH sequence (TR13ms, TE4.9ms, FOV256mm, BW230Hz) was performed in each position once the test sphere was rotated. Following data collection the knees were assessed by a specialist orthopaedic veterinarian who gave a pathological diagnosis having dissected and photographed the joint. The raw 3D T1 FLASH volumes were registered and aligned then compared to identify large variations of signal intensity. Segmentation using a thresholding technique identified voxels containing collagen. For each collagen-rich voxel the orientation vector was computed using Szeverenyi and Bydder’s5 method. Each orientation vector reflects the net effect of all the fibers comprised within a voxel. The assembly of all unit vectors represents the fiber orientation map and was visualised in ParaView6 using streamlines (figure 1). The Alignment Index (AI) is defined as a ratio of the fraction of orientations within 20° (solid angle) centred in that direction to the same fraction in a random (flat) case7. By computing AI for a regular gridded orientation space we are able to visualise differences in AI on a hemisphere (figure 2). AI was normalised so that AI=0 indicates isotropic collagen alignment. Increasing AI values indicate increasingly aligned structures: AI=1 indicates that all collagen fibers are orientated within the cone of 20° centred at the selected direction.Results
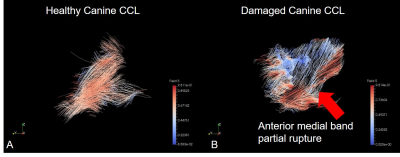
Of the ten canine knees scanned, two had ligament pathology identified on PM. Dogs have a cranial caudal ligament (CCL) which is similar to the anterior cruciate ligament (ACL) in humans, seen in figure 3A as a healthy shiny ligament. It is composed of two bundles, an anteriomedial (AM) bundle and a posteriolateral (PL) bundle. Two canine knees were damaged with partial CCL tears, the PL bundle was intact but the AM bundle was torn (figure 3B).
A ParaView6 streamline visualisation of the CCL collagen tracts of a healthy and damaged canine knee are shown in Figure 1. The fiber tracts are continuous in the healthy canine knee (A) with no disruption of the ligament. In (B) the AM bundle fibers (red) are discontinuous and the PL bundle fibers (blue, behind) are continuous which one would expect in a partially torn CCL.
The AI for a healthy and a damaged CCL is shown in Figure 2. This visualises a difference in the collagen fiber alignment between the healthy (A) and damaged (B) CCL. The damaged AM bundle is visualised as a more diffuse spread of less aligned fibers compared to the more concentrated and aligned PL fiber bundles.
Discussion
CCL disease is a gradual degeneration of the ligament extracellular matrix (ECM) leading to ligament rupture8. Certain breeds of dog (e.g. Labrador Retreiver, Rottweiler, Newfoundland, Boxers) are at increased risk of developing CCL disease9. Human partial ACL tears are extremely difficult to diagnose using traditional MR imaging which provides no functional assessment of the remaining portion10. However, using our magic angle imaging technique the partial ligament rupture is clearly visualised. The AI provides a quantified measure of the alignment of the collagen fiber bundles.Conclusion
This study demonstrates the first visualisation of a canine CCL partial
tear using magic angle imaging. Combined
with AI, our scanning technique offers a tool to visualise and quantify changes
in collagen fiber orientation. We have
demonstrated that MRI can be used to improve our ability to diagnose and
quantify a partial ligament tear in the knee. Acknowledgements
This work was supported by the National Institute for Health Research (NIHR) Invention for Innovation (i4i) under Grant II-LA-1111-20005. We are grateful to Charing Cross Hospital MRI department and Imaging Committee for the kind use of the Siemens 3T Verio. We would also like to thank the RVC PM technician Richard Prior for his assistance with canine knee collection.References
1 Bydder, M. Rahal, A. Fullerton, G.D. and Bydder, G.M. 2007. The magic angle effect: a source of artefact, determinant of images contrast and technique for imaging. Journal of Magnetic Resonance Imaging, 25, 290-300.
2 Chang, E.Y. Szeverenyi, N.M. Statum, S. Chung, C.B. 2014. Rotator cuff tendon ultrastructure assessment with reduced-orientation dipolar anisotropy fiber imaging. AJR, 202, W376-378.
3 Fullerton, G.D. & Rahal, A. 2007. Collagen structure: the molecular source of the tendon magic angle effect. Journal of Magnetic Resonance Imaging, 25, 345-361.
4 Spriet, M. et al. 2012. Magic angle magnetic resonance imaging of diode laser induced and naturally occurring lesions in equine tendons. Veterinary Radiology and Ultrasound. 53(4): 394-401.
5 Szeverenyi, N.M. & Bydder, G.M. 2011. Dipolar anisotropy fiber imaging in a goat knee meniscus. Magnetic Resonance in Medicine.65:463–470.
6 Ahrens, J., Geveci, B. & Law, C. 2005. ParaView: An End-User Tool for Large Data Visualization, Visualization Handbook, Elsevier, Burlington, Massachusetts, USA.
7 Chappell, et al. 2017. The alignment index: a new method to analyse collagen fibre orientation distribution in the knee. EORS 2017 proceedings
8 Comerford, E.J. et al. 2011. Update on the aetiopathogenesis of canine cranial cruciate ligament disease. Vet Comp Orthop Traumatol. 24(2):91-98
9 Taylor-Brown. F.E. et al. 2015. Epidemiology of cranial cruciate ligament diseases diagnosis in dogs attending primary-care veterinary practices in England. Veterinary Surgery. 44: 777-783.
10 Temponi, E.F. et al. 2015. Partial tear of the anterior cruciate ligament: diagnosis and treatment. Rev Bras Orthop. 50(1):9-15
Figures