1036
A Pilot Study of Correlations Between Mean Diffusivity and Drug Release Over Time For an Implantable Drug Delivery System1Basic Medical Sciences, Purdue University, West Lafayette, IN, United States, 2Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 3Electrical and Computer Engineering, Purdue University, West Lafayette, IN, United States
Synopsis
In situ forming implants (ISFI) allow for precise placement of a drug eluting depot into the body, using a simple injection. However, the properties of the injection sites can alter the drug release profile in situ. Diffusion weighted imaging (DWI) provides a method for non-invasively and nondestructively tracking changes in the implant diffusivity in situ. Using DWI, we have characterized the diffusivity profile of ISFIs made using a poly(lactic-co-glycolic) acid polymer, loaded with the mock drug fluorescein. The ability to characterize the diffusivity profile of implants in situ, will provide the necessary tools for improving the rational design of implants.
Introduction
Injectable drug delivery systems, such as ISFIs, provide a means of maintaining therapeutic levels of drugs over time, without the need for taking multiple pills.1 Phase-sensitive ISFIs are formed when a drug is mixed with a biodegradable polymer that is dissolved in a biocompatible, water miscible solvent. When the polymer solution contacts an aqueous environment, counter-transport of both solvent and water occurs, causing the polymer to precipitate out of solution. The rate of solvent/nonsolvent exchange controls the implant microstructure, and ultimately the diffusivity of the implant.2-4 By tracking changes in the implant diffusivity over time in situ, we can characterize the effects that the injection site has on the mass of drug released from the implant both in vitro and in vivo. Diffusion-weighted imaging (DWI) provides a means to both visualize and quantify changes in implant diffusivity noninvasively. This imaging data can then be correlated to the cumulative release data to drive design decisions for implant formulation.Methods
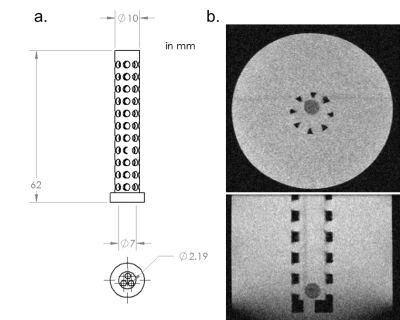
Drug Implant Synthesis: Poly(lactic-co-glycolic) acid (PLGA), N-methyl-2-pyrrolidone (NMP), and sodium fluorescein were combined in a 39:60:1 mass ratio. Fluorescein was dissolved in NMP and the PLGA polymer was then added to the solution. 60 μL of this solution was injected into 10 mL of Millipore water in order to form the solid implant (Fig. 1). An implant was prepared for each time point. In addition, the release of fluorescein from the implant was measured over time by taking samples at 0.25, 0.5, 1, 2, 4, 6, 24, 48, 72, and 96 hours post-injection. The fluorescence of this sample was quantified using a SpectraMax M5 microplate reader, and results were compared to a standard curve to obtain the mass of fluorescein released.
DWI: To measure the mean diffusivity (MD) of drug out of the polymer, imaging was conducted in phantom. The phantom contains a custom 3D printed part inserted into a 60 mL plastic bottle which ensures the implant is centered in the phantom bottle during imaging (Fig. 2). The PLGA implant, containing fluorescein, was placed into the 3D printed tube and the bottle was filled with RODI water. Imaging was conducted using a Bruker BioSpec 70/30 USR 7T Preclinical MRI system and Bruker rat head/mouse body RF RES 300 1H 075/040 QSN TR volume coil. A standard diffusion-weighted spin echo protocol was utilized (TE=17.5 ms, TR=2500 ms, FOV=45x45 mm2, slice thickness=0.80 mm, b=0,1000 s/m2). Implants synthesized at various time points (4, 5.5, 8, 26, 50, 74, and 101 hours) were imaged during one scanning session.
Results
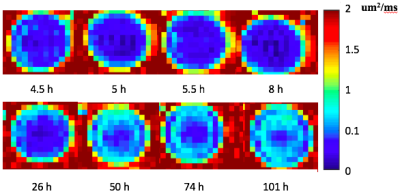
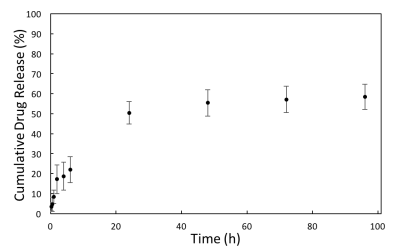
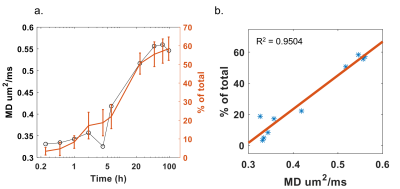
Figure 3 illustrates the apparent diffusion coefficient (ADC) maps of the drug implant over time, from 1 to 101 hours. Higher diffusivity appears at the periphery of the drug implant during hours 1-8, then gradually decreases. Figure 4 shows cumulative fluorescein release from the drug delivery polymer in situ over the 96 hours following injection. There is an initial burst release during the first 24 hours during which approximately 50% of the drug is released. After this time, the drug release is controlled by diffusion through the polymer resulting in 58% of the drug released after 96 hours. Figure 5a shows the plot of MD and cumulative fluorescein release out of the drug delivery polymer in situ within 101 hours post-injection. Similar drug release patterns are observable between these experiments. Figure 5b shows a 95% correlation between in situ drug release and MD.Discussion
As can be seen, the MD profile correlates well with the cumulative drug release profile (Fig. 5a). In the initial burst release phase, the solvent is exiting the polymer implant as water enters, releasing large amounts of drug in the process which corresponds to increasing MD. Then, as the outer shell forms, drug release slows down and becomes controlled by diffusion through the polymer. At this point, the increasing diffusivity values also level off. This trend is also represented in Figure 5b, which verifies the accuracy of DWI to measure drug release over time. The use of MD as a noninvasive metric for drug release can have applications for monitoring implants in vivo.Conclusion
Drug delivery implants provide a means to improve patient compliance and approval. However, no methods exist to examine the release profile of various drugs in vivo, over time. The data presented here provides evidence that DWI can be used to noninvasively measure MD of drug across the implant polymer in phantoms, over time. This method can be used to assess the time course of drug release, providing a means to test treatment efficacy.Acknowledgements
We would like to thank Dr. Gregory Tamer for assistance with the Bruker 7T MRI system and Dr. Chris Flask for helpful discussions regarding diffusion-weighted imaging on the Bruker BioSpec scanner. This work is supported by NIH funding (grant R00CA198929).References
1. Dunn R, English J, Cowsar D, Vanderbilt D. (1990). United States Utility Patent NO 4,938,763 A. Biodegradable in situ forming implants and methods of producing the same. Washington D.C., USA: United States Patent Office.
2. Solorio L, Babin B, Patel R, Mach J, Azar N, Exner A. Noninvasive characterization of in situ forming implants using diagnostic ultrasound. Journal of Controlled Release. 2010;143(2):183-190.
3. Solorio L, Olear A, Hamilton J, Patel R, Beiswenger A, Wallace J, Zhou H, Exner A. Noninvasive characterization of the effect of varying PLGA molecular weight blends on in situ forming implant behavior using ultrasound imaging. Theranostics. 2012;(2):1064-1077.
4. Solorio L, Olear A, Zhou H, Beiswenger A, Exner A. Effect of cargo properties on in situ forming implant behavior determined by noninvasive ultrasound imaging. Drug Delivery and Translational Research 2012;(2):45-55.
Figures