1027
Development of a diffusion-weighted SSFP acquisition and processing pipeline to quantify the diffusion properties of the post-mortem ALS brain at 7T1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Department of Radiology, University of Chicago, Chicago, IL, United States, 3Clinical Neurology, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 4Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom
Synopsis
An acquisition and processing pipeline is outlined to quantify the diffusion properties of post-mortem brain samples from diffusion-weighted steady-state free precession (dwSSFP), as part of an ongoing project examining the impact of amyotrophic lateral sclerosis (ALS) on the human brain. Preliminary results are presented comparing the post-mortem diffusion properties of control brains and patients diagnosed with ALS over the corpus callosum. Clear trends are observed within the results, with a maximum deviation between control and ALS brains observed within callosal regions connecting the motor cortices in the two hemispheres.
Introduction
Diffusion-weighted imaging of brain tissue provides researchers with an invaluable opportunity to compare data to microscopy techniques that directly quantify microstructure. Moreover, scanning of whole brains enables characterisation of neuropathological spread. Reductions in both diffusivity and T2 lead to competing demands in diffusion-weighted spin-echo1. The combination of short echo-time to accommodate reduced T2 and high b-value to counter lower diffusivity can only be achieved with powerful gradient coils, typically only available on rodent scanners which cannot accommodate whole human brains. Diffusion-weighted steady-state free precession (dwSSFP) is well suited to the T2 and ADC of post-mortem tissue1,2. However, the dwSSFP signal has complex dependencies on T1, T2 and B13, making quantification challenging. An acquisition and processing pipeline was developed to quantify the diffusion properties of post-mortem brain samples from dwSSFP at 7T. This work forms part of an ongoing project relating MRI to histopathology in patients diagnosed with the adult neurodegenerative disorder of the motor system amyotrophic lateral sclerosis (ALS). Preliminary results are presented comparing the diffusion properties of control and ALS brains over the corpus callosum.Method
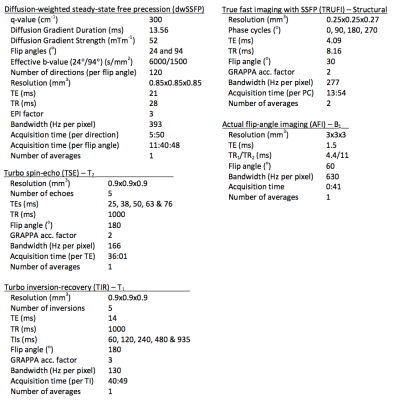
Formalin-fixed, post-mortem whole brain samples were immersed in 3MTM Fluorinert (FC-3283), and placed inside a custom-designed holder, constructed to maximize field homogeneity and ensure that samples were reliably positioned within the scanner. Samples were scanned at 7T (Siemens, 1Tx/32Rx coil) using a multi-modal imaging protocol, including dwSSFP scans and quantitative parameters relevant to the dwSSFP signal (T1, T2, B1). dwSSFP was acquired with a dual flip angle protocol designed to account for the B1 field inhomogeneity at 7T4. A structural scan was additionally obtained to assist in coregistration and generate a mask of the post-mortem tissue. Details of the acquisition protocol are outlined in Figure 1.
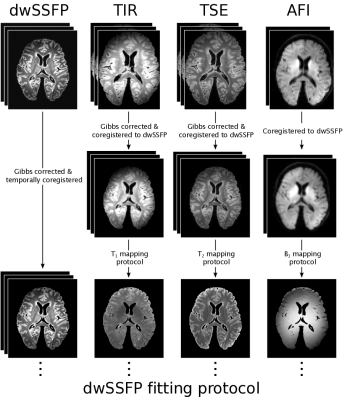
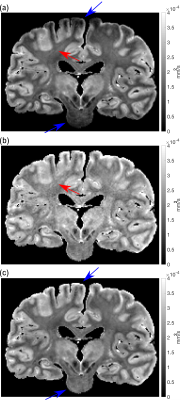
Figure 2 displays the initial processing pipeline for each post-mortem brain. The tensor model was fit to the dwSSFP data using a modified version of DTIFIT5 that incorporates the dwSSFP signal equation, including T1, T2 and B16. This implementation further accounts for dual flip-angle dwSSFP, in which restriction causes the signal to deviate from the Buxton model7. The eigenvalue maps ($$$\lambda_{1,2,3}$$$) were extrapolated to a common apparent flip angle to account for B1 inhomogeneity (Figure 3). Finally, fractional anisotropy (FA) and mean diffusivity (MD) maps were generated from the corrected $$$\lambda_{1,2,3}$$$ maps.
Diffusion properties of control and ALS brains were compared over the corpus callosum. The derived fractional anisotropy (FA) maps were coregistered to a standard-space FA template (FMRIB58_FA)5 via ANTs8. A mask of the corpus callosum was generated from the Jülich atlas5,9. The corpus callosum was split into 5 distinct areas associated with specific fibre projections10. Small ROIs were hand-drawn within the centre of these areas for analysis. All results were normalised to the mean of the splenium of the callosum, previously proposed as a control region with little pathological burden in ALS11. Results presented here are for nine post-mortem brain samples (three control and six ALS). Differences in the normalised FA, MD, axial and radial diffusivity between the control and ALS brains were assessed with a two-tailed, family-wise error rate (FWER) corrected t-test using PALM12.Results
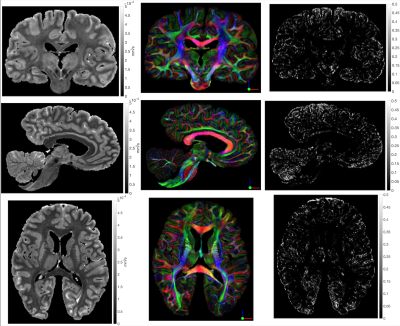
Figure 4 shows the MD, FA-modulated $$$\vec{V}_{1}$$$ and fibre-orientation uncertainty maps of a post-mortem ALS brain. The MD map depicts clear anatomical information, revealing distinctive grey-white matter contrast throughout the sample. The FA-modulated $$$\vec{V}_{1}$$$ displays well-defined orientation information, with low uncertainty observed throughout white-matter.
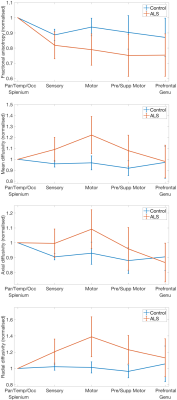
Figure 5 shows the variation of the FA, MD and axial/radial
diffusivity within the corpus callosum. No significant differences (p<0.05) were observed between the control and ALS brains examined (MD of the motor region does show statistical significance without FWER correction). However clear trends are observed, with a maximum deviation between
control and ALS brains within the central regions
of the corpus callosum, peaking in the callosal regions that connect motor
cortices in the two hemispheres10
Conclusion
The processing-pipeline presented here generates high-quality diffusion metrics within whole, fixed, post-mortem human brain samples. Preliminary analysis observes a maximal deviation in the diffusion properties of the callosal regions related to the motor system but not the genu and splenium of the callosum, consistent with both previous MRI studies and our knowledge of pathological burden.Future Directions
Histology is being performed on all datasets within regions
associated with the
proposed pathological
staging system
of ALS13 , with the ambition to link the
quantitative metrics derived from the multi-modal MRI datasets with
quantitative information about the tissue microstructure and pathology. Future
work will continue in processing additional datasets, improving the dwSSFP
protocol and forming a more comprehensive analysis of the ALS-staging regions.Acknowledgements
No acknowledgement found.References
1 McNab, Jennifer A., et al. "High resolution diffusion-weighted imaging in fixed human brain using diffusion-weighted steady state free precession." Neuroimage 46.3 (2009): 775-785.
2 Foxley, Sean, et al. "Improving diffusion-weighted imaging of post-mortem human brains: SSFP at 7T." Neuroimage 102 (2014): 579-589.
3 McNab, Jennifer A., and Karla L. Miller. "Steady‐state diffusion‐weighted imaging: theory, acquisition and analysis." NMR in Biomedicine 23.7 (2010): 781-793.
4 Foxley, Sean, et al. “Correcting for B1 inhomogeneities in post-mortem DWSSFP human brain data at 7T using multiple flip angles.” 22th Proc. Intl. Soc. Mag. Reson. Med., 2014: #4438.
5 Jenkinson, Mark, et al. "Fsl." Neuroimage 62.2 (2012): 782-790.
6 Buxton, Richard B. "The diffusion sensitivity of fast steady‐state free precession imaging." Magnetic resonance in medicine 29.2 (1993): 235-243.
7 Jbabdi, Saad, et al. “Modelling multiple flip angle diffusion weighted SSFP data.” 23rd Proc. Intl. Soc. Mag. Reson. Med., 2015: #2928.
8 Avants, Brian B., et al. "The Insight ToolKit image registration framework." Frontiers in neuroinformatics 8 (2014).
9 Eickhoff, Simon B., et al. "A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data." Neuroimage 25.4 (2005): 1325-1335.
10 Hofer, Sabine, and Jens Frahm. "Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging." Neuroimage 32.3 (2006): 989-994.
11 Cardenas, Agustin M., et al. "Pathology of callosal damage in ALS: An ex-vivo, 7T diffusion tensor MRI study." NeuroImage: Clinical 15 (2017): 200-208.
12 Winkler, Anderson M., et al. "Permutation inference for the general linear model." Neuroimage 92 (2014): 381-397.
13 Tan, Rachel H., et al. "TDP-43 proteinopathies: pathological identification of brain regions differentiating clinical phenotypes." Brain 138.10 (2015): 3110-3122.
14 Yarnykh, Vasily L. "Actual flip‐angle imaging in the pulsed steady state: a method for rapid three‐dimensional mapping of the transmitted radiofrequency field." Magnetic resonance in Medicine 57.1 (2007): 192-200.
15
Kellner, Elias, et al. "Gibbs‐ringing artifact
removal based on local subvoxel‐shifts." Magnetic resonance in medicine
76.5 (2016): 1574-1581.
Figures