1021
Diffusion-weighted in vivo imaging with ≤100 µm resolution: Principles and applications to ADC mapping of pregnant mice1Departments of Chemical and Biological Physics, Weizmann Institute of Science, Rehovot, Israel, 2Department of Electrical Engineering and Computer Science, UC Berkeley, Berkeley, CA, United States
Synopsis
Although a major advantage of SPatiotemporal ENcoding (SPEN) vs EPI is a higher immunity to artifacts, it suffers –as all single-shot experiments– from resolution and SNR limitations. These can be dealt by multi-scan interleaving, which unlike EPI counterparts leads to independent low-resolution images free from aliasing artifacts. We present an acquisition and processing protocol that reconstructs from these data a composite image free from hardware or motional imperfections, possessing unprecedented resolution. The power of this self-referenced method is demonstrated with in vivo ADC maps of brains, kidneys and fetal organs in pregnant mice, possessing resolutions in the 78-230 µm range.
INTRODUCTION
Diffusion provides unique in vivo insight under normal and pathological conditions, helping to reveal structures and microstructures. The pulsed gradients used in these measurements make multi-scan experiments incompatible in vivo motions, demanding single-shot imaging methods like EPI’s quality, however, is compromised by field distortions. SPatiotemporal ENcoding (SPEN) is a single-shot method enabling the use of larger gradients, hence providing higher immunity to these heterogeneities.[1] Still, both single-shot EPI and SPEN suffer from limited resolution and compromised sensitivities. Multi-shot interleaving can bypass these, yet in EPI this comes at the expense of reintroducing sensitivity to motions. Not so necessarily in SPEN, where interleaving will still comprise the acquisition of low-resolution images in every scan. This paper introduces new acquisition and processing methods that exploit this, and demonstrates their potential with ADC maps acquired in vivo for olfactory bulbs and kidneys in naïve mice and for organs in pregnant mice –including fetal brain and placental diffusivity maps.METHODS
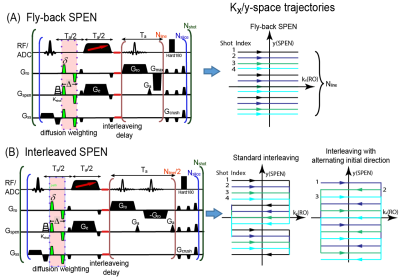
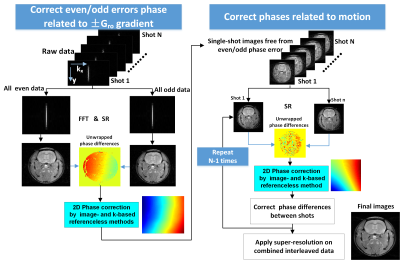
Like multi-shot EPI, interleaved SPEN[2] will be affected by two main artifacts: one arises from gradient imbalances during the readout train that translates in SPEN into even/odd “stripes”[3, 4]; another arises from motions between shots and leads to phase errors. Interleaved SPEN, however, delivers low-resolution images for every even/odd kRO set within every shot; based on this, Figure 1 introduces three kinds of interleaving schemes capable of accounting for all artifacts without demanding navigator scans. All schemes contain a variable “kshot” gradient and delay that shifts the spatial positions of interleaved shots along the SPEN (y) direction. Figure1A is a fly-back scheme where data are only acquired during positive readout gradients, freeing images from even/odd distortions at the expense of efficiency. The other two schemes utilize positive and negative readout gradients, but differ in their kx/y-space trajectories: one employs standard interleaving, the other interleaves while alternating the initial kRO directions. In all cases intra- and inter-shot inconsistencies are corrected using information arising from each set itself, but the scheme with alternating initial kRO directions is best to map the phase distortions of even/odd readouts, due to its higher point density along the SPEN-domain. Figure 2 shows a processing algorithm developed for removing from these data all phase artifacts. SPEN’s non-Fourier nature and lack of image aliasing allows us to perform this for every even/odd set within every shot. These corrections involved equalizing all phases using second-order polynomial fits in (x,y). First a coarse correction was done using super-resolved (SR) SPEN images arising from individual even/odd sets. Subsequently a finer correction was done on these SR SPEN images, which would still showing striping as a result of residual imperfections. Upon FT these stripes translate into high-frequency ky-space echoes; the second, finer correction involved finding the phases that minimized these spurious echoes. To assess this strategy’s ability to provide artifact-free high-resolution ADC maps, data were collected on a 7T/120mm horizontal Agilent MRI scanner and quadrature Millipede® coil, and comparisons run against single-shot SPEN and EPI counterparts. All abdominal measurements were respiration-only synchronized.RESULTS & DISCUSSION
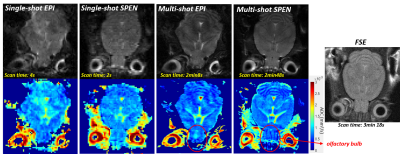
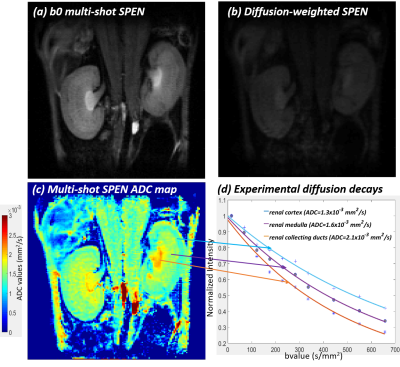
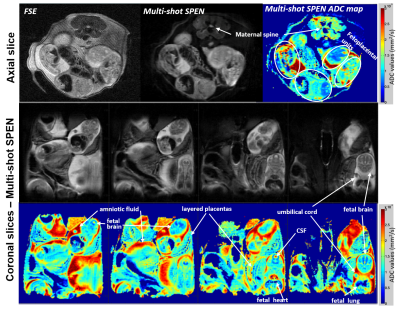
Figure 3 compares single- and multi-shot b0 and ADC images collected on a live mouse’s brain. Notice the good agreement between the low (single-shot) and high resolution (multi-shot) ADC maps, which in the latter case access the sinuses-challenged olfactory bulb without distortions at a 79µm in-plane resolution. Figures 4 and 5 center on in vivo abdominal images of pregnant and naïve mice. The kidney’s ADC map in Figure 4 clearly reveals the different behavior of the cortex, medulla, collecting ducts and collecting papilla layers. These layers have been observed in anatomical mice images, but to our knowledge it is the first time they are revealed by ADC maps [5]. The pregnant mouse’s ADC map also reveals for the first time multiple fetoplacental features, including a clear delineation of fetal brain hemispheres with distinct CSF and white matter values, fetal organs including lung and heart, fast-diffusing amniotic fluid, distinct placental layers (maternal, fetal, trophoblasts), diffusing fluid in the umbilical cord, and the maternal spine.CONCLUSION
Interleaved acquisition and processing schemes that exploit SPEN’s image-based, aliasing-free nature, allowed us to reconstruct diffusion images with unprecedented resolution from challenging regions. The methods’ abilities to withstand heterogeneities and motions and to zoom without folding enabled in vivo ADC mapping of the olfactory bulb at high resolution, of various layers in the kidney structure, of fetal mouse brains, and of layers in the maternal placentas. The biological insight that this provides is under study.Acknowledgements
We are grateful to Dr. Zhiyong Zhang and Dr. Amir Seginer for valuable discussions.Financial support came from the NIH human placenta project (R01HD086323), Minerva funding (#712277), the Kimmel Institute for Magnetic Resonance and the generosity of the Perlman Family Foundation.References
1. Tal, A. and L. Frydman, Single-scan multidimensional magnetic resonance. Prog Nucl Magn Reson Spectrosc, 2010. 57(3): p. 241-92.
2. Schmidt, R., A. Seginer, and L. Frydman, Interleaved multishot imaging by spatiotemporal encoding: A fast, self-referenced method for high-definition diffusion and functional MRI. Magn Reson Med, 2016. 75(5): p. 1935-48.
3. Ben-Eliezer, N., M. Irani, and L. Frydman, Super-resolved spatially encoded single-scan 2D MRI. Magn Reson Med, 2010. 63(6): p. 1594-600.
4. Seginer, A., et al., Referenceless reconstruction of spatiotemporally encoded imaging data: principles and applications to real-time MRI. Magn Reson Med, 2014. 72(6): p. 1687-95.
5. Hueper, K., et al., Multiparametric Functional MRI: Non-Invasive Imaging of Inflammation and Edema Formation after Kidney Transplantation in Mice. PLoS One, 2016. 11(9): p. e0162705.
Figures