1015
Dopamine Release and Neural Activation Differs between Control and MDD during Reward Anticipation: a Simultaneous [11C]Raclopride PET-fMRI Study1Radiological Sciences Laboratory, Department of Radiology, Stanford University, Palo Alto, CA, United States, 2Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, China, 3Harvard-MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 4A. A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 5Center for Social and Affective Neuroscience, Linköping University, Linköping, Sweden, 6Department of Psychology, Stanford University, Palo Alto, CA, United States, 7Applied Science Lab, GE Healthcare, Menlo Park, CA, United States
Synopsis
The interaction between the midbrain dopaminergic system and the striatum is implicated in reward processing; it is still unknown, however, how this interaction is altered in Major Depressive Disorder (MDD). In the current study, we compared coupling of dopamine release/binding and neural activity in adults diagnosed with MDD and healthy controls (CTLs) during a monetary incentive delay (MID) task using simultaneous $$$[^{11}C]$$$Raclopride PET and fMRI. We obtained significant correlations in CTLs but not in MDD patients, indicating that the decoupling of dopaminergic system and striatum, especially nucleus accumbens, may play a vital role in the pathophysiology of MDD.
Introduction
The interaction between the midbrain dopaminergic system and the striatum, especially the nucleus accumbens (NAcc), is a crucial component of reward processing. It has been reported that the dopamine release/binding at D2 receptors measured by PET couples with neural activity inferred by BOLD fMRI in normal humans by separate PET and fMRI scans1. Adults diagnosed with Major Depressive Disorder (MDD) have shown reduced neural activation during reward processing2; however, it is still unknown whether this altered activation is related to the decoupling of dopamine release/binding and neural activation. In the current study, we conducted simultaneous $$$[^{11}C]$$$Raclopride PET-fMRI to explore the relation of dopamine release/binding and neural activity between MDD and healthy controls (CTLs) during a monetary incentive delay (MID) task3. We hypothesized that disrupted coupling would be observed in the MDD group.Methods
Participants: Twelve MDD participants (11 unmedicated; 7 female, 5 male; average age = 32. 22 years) and thirteen CTL subjects (8 female, 5 male; average age = 32.57 years) were recruited for the study. Written informed consent was obtained from participants.
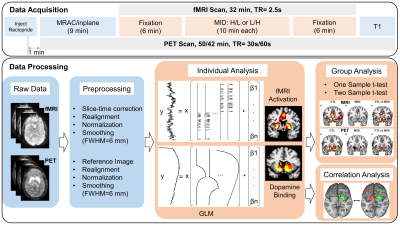
Data Acquisition: All participants underwent a simultaneous PET-MRI examination (Fig.1) on a time-of-flight (TOF) PET-MRI scanner (SIGNA PET-MRI; GE Medical Systems, USA). PET scanning took place for the entire 42-minute /50-minute scanning session. A bolus injection of $$$[^{11}C]$$$Raclopride was administered one minute after the PET scan started, and we initiated a 32-minute-long fMRI scan 9 min after the injection to allow for the uptake of the radiotracer. During the scan, participants performed two counterbalanced MID tasks (10-min each) at different stake levels (low: $1; high: $5). The experiment included 4 task conditions: high/low stake reward/punishment and 4 baseline conditions for each task condition.
Preprocessing and data analysis (Fig.1) were implemented in DPABI (http://rfmri.org/DPARSF), SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) and self-developed MATLAB code.
fMRI Data Analysis: A routine preprocessing pipeline was applied to the fMRI data. Percent signal change was calculated with respect to the mean activity over the entire task session. Preprocessed data were entered into the GLM at the individual level, activation of anticipation (~2.5s) of low/high stake reward/punishment versus no reward/no punishment contrasts was estimated.
PET Data Analysis: We averaged the 2-8 minutes pre-task PET data to generate a reference image for a rigid body realignment across different timepoints, and then co-registered all PET images to the mean fMRI image. PET images were normalized and smoothed with the same parameters as for fMRI. As with fMRI, PET data were submitted to a GLM, the integral of two exponential functions with time constant of 3 min derived from preliminary investigation was applied to model dopamine release. The mean striatum timecourse was fitted by a gamma variate function, and was applied to regress out the uptake and washing out of the radiotracer. This method can efficiently detect the activation from data simulated by classical SRTM kinetic modeling4.
Group Analysis: For both fMRI and PET analyses, a one-sample t-test was conducted to generate the group average activation/dopamine binding, and a two-sample t-test was conducted for group comparison between MDD and CTL groups. The statistical significance criterion was set at p<0.05, FDR-corrected for multiple comparisons.
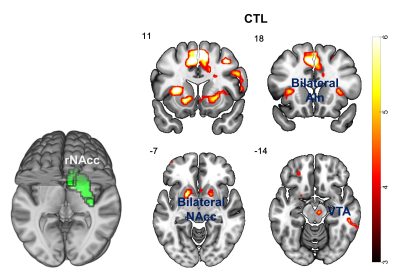
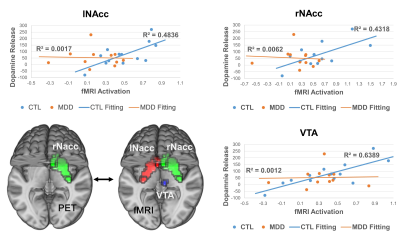
Correlation Analysis: Dopamine binding in NAcc was extracted using a well-established template5 (Fig.4), and correlated to the fMRI activation voxel by voxel for CTL and MDD groups separately.
Results
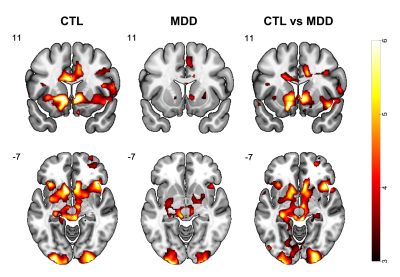
fMRI: Anticipation of both reward versus no-reward and punishment versus no-punishment induced activation in the striatum, including the NAcc. The high-reward versus no-reward contrast induced the most significant activation among the 4 conditions and is shown as an example (Fig.2). Group analysis revealed an overall reduced activation in MDD compared with CTL in the striatum region (Fig.2).
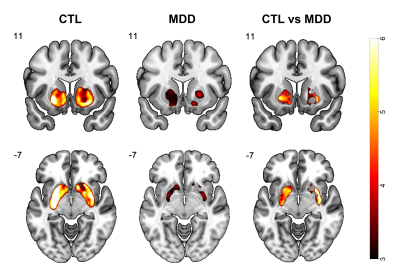
PET: During the MID task, increased dopamine binding in the striatum, including NAcc, was observed in both the CTL and MDD groups, and was stronger in the CTL group (Fig.3), which is consistent with the fMRI findings.
Correlation: For the CTL group, dopamine binding in rNAcc was correlated with fMRI activation of NAcc (lNAcc: p=0.007, rNAcc: p=0.015), ventral tegmental area (VTA, p=0.001), etc. (Fig.4). However, no significant correlation was observed for the MDD group. The relation between dopamine binding in rNAcc and fMRI activation in bilateral NAcc, VTA is shown in Fig.5.
Discussion and Conclusion
We conducted a concurrent $$$[^{11}C]$$$Raclopride PET-fMRI study to explore the relation between dopamine release/binding and neural activity in the reward system for MDD. As we hypothesized, significant correlation for the CTL group was observed, while the MDD group showed no correlation, indicating that the disconnection between dopaminergic system and striatum may be a key factor in the development of MDD. Thus, the combination of complementary PET and fMRI may provide valuable information in understanding the pathophysiology of psychiatric disorders.Acknowledgements
We thank Prof. Brian Knutson for assistance with the task design, Matthew Sacchet, Hershel Mehta and Christina Schreiner for scanning, Prof. Lihong Wang and Christine Law for valuable discussions. This work was supported in part by NIH EB015891 (GG, JC) and Weston Havens Foundation (IHG, JPH).References
1. Schott, B.H., et al., Mesolimbic Functional Magnetic Resonance Imaging Activations during Reward Anticipation Correlate with Reward-Related Ventral Striatal Dopamine Release. Journal of Neuroscience, 2008. 28(52): p. 14311-14319.
2. Whitton, A.E., M.T. Treadway, and D.A. Pizzagalli, Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current Opinion in Psychiatry, 2015. 28(1): p. 7-12.
3. Knutson, B., et al., Neural responses to monetary incentives in major depression. Biological Psychiatry, 2008. 63(7): p. 686-692.
4. Fuyixue Wang, et al., A Preliminary Study of Major Depressive Disorder Using Simultaneous PET/fMRI with Two MID Tasks in a Single Scan. in ISMRM. 2016.
5. Fan, L.Z., et al., The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cerebral Cortex, 2016. 26(8): p. 3508-3526.
Figures