1014
Development of Multimodal Neuroimaging Technology at Ultrahigh Field for Studying Brain Function from Cell to Network1Center of Magnetic Resonance Research, Department of Radiology, University of Minnesota Medical School, Minneapolis, MN, United States, 2School of Electrical and Computer Engineering, Department of Biological Sciences, Purdue University, West Lafayette, IN, United States
Synopsis
Functional MRI based on blood oxygenation level dependent (BOLD) contrast has become an indispensable method for mapping effective and functional connectivity. However, subject to intrinsic resolution limit, fMRI cannot be used to directly explore cellular and neurovascular mechanisms underlying BOLD contrast at microscopic scale. To overcome this obstacle, a novel integrated two-photon fluorescence microscopy (TPM)-MRI system at ultrahigh field of 16.4 tesla is proposed and developed. This multimodal neuroimaging tool will enable noninvasive and simultaneous measurement of neurophysiological change and fMRI signal with unprecedented resolution and sensitivity, thus, bridging microscopic neural and vascular dynamics with macroscopic brain networks.
Introduction
Various neuroimaging techniques exist for brain function study, among which functional magnetic resonance imaging (fMRI) based on blood oxygenation level dependent (BOLD) contrast has earned a prominent position in neuroscience for mapping neuron activation noninvasively across whole brain either during task or at resting state. Benefit from ultrahigh magnetic field, fMRI can map activations down to neural-computational units including cortical layers [1] and functional columns [2-3] in the brain cortex or subcortex. However, due to the intrinsic physical mechanism, fMRI cannot reach adequate specificity and sensitivity to decipher neuronal functions at the cellular and micro-vascular scales. Comparatively, two-photon fluorescence microscopy (TPM), though suffering from a limited brain coverage, is a powerful tool in revealing cellular mechanisms of single neuron and microcircuit functions [4]. Relying on its extremely high spatiotemporal resolution (<1 μm), TPM can discern neurons, dendritic spines, astrocytes, capillaries, microvessels and monitor their functional activities and dynamics. To be able to comprehensively assess neuronal activities from cellular, neural and vascular dynamics to brain networks, we addressed multiple technical challenges and paved the way to the first MRI-compatible TPM integrated with ultrahigh MR-based imaging technologies at 16.4T [5].Materials and Methods
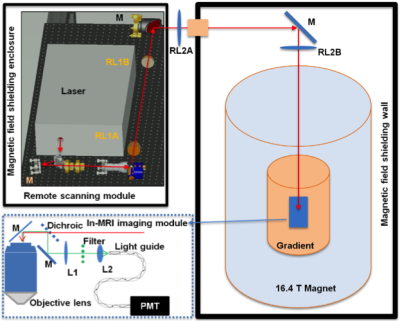
Experiment setup: As most parts of the optical system cannot function properly in high magnetic field, thus, a long-distance (> 9 m) remote TPM-MRI multimodal imaging system was designed. This novel TPM system consisted of a remote laser scanning system located outside the 16.4T magnet room and an in-MRI optical imaging module inside the 16.4T magnet (Fig. 1). Components of the in-MRI module were non-magnetic and any delicate electronic devices and actuators used in the remote laser scanning system were magnetically shielded to near the earth field strength.
Imaging performance: Based on the above experimental setup, optical imaging performance at ultrahigh magnetic field 16.4T was tested using (1) fluorescence beads of 1μm in diameter as the standard imaging sample, (2) fixed CX3CR1 mouse brain, and (3) in vivo transgenic mouse brain with and without MRI gradients and radiofrequency pulses on, respectively. A more detailed description of the experiment setup and imaging subjects can be found in the reference [5]. The animal procedures and experiments were conducted under the protocols approved by the Institutional Animal Care and Use Committee of University of Minnesota and Purdue University.
Results
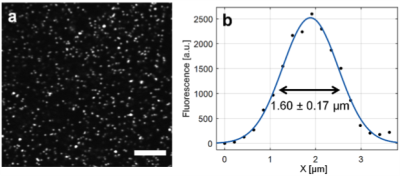
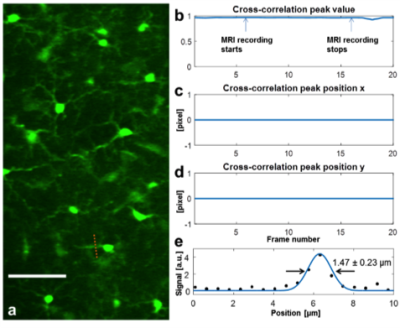
The long-distance remote TPM-MRI multimodal imaging system was installed and tested on site in the 16.4T magnetic field. In the TPM imaging spatial resolution test inside of 16.4T magnet, the observed TPM image and FWHM of the standard beads (Fig. 2a) and microglia cells in the fixed mouse brain (Fig. 3a) was 1.60 ± 0.17 μm (n = 10) (Fig. 2b) and 1.47 ± 0.23 μm (n = 5) (Fig. 3e), respectively, which were very close to the best diffraction-limited resolution with the current setup. The results indicate the TPM system can deliver high imaging quality at such high magnetic field, with minimal aberrance and distortion even when the MR gradients were switched on-and-off (Figs. 3b-d). We have recently conducted another pilot in vivo study using a transgenic mouse brain, demonstrated for the first time in imaging individual red blood cells moving in capillaries inside the 16.4T magnet.Discussion and Conclusion
Our preliminary results show that: 1) the long-distance remote TPM-MRI system can effectively protect the TPM components from the strong magnetic field; 2) the long-distance relay can enable near diffraction-limit imaging performance; 3) the novel TPM imaging system is compatible with the ultrahigh-field MRI system without interference from the MRI operation. Based on these results, it is evident that a substituted brass free objective lens which can be customized and built by a vendor will enable simultaneous fMRI and TPM measurement of various critical neurological parameters inside the 16.4T magnet with unprecedented spatiotemporal resolution and scale.
In summary, this study proved the concept of building a cutting-edge MRI-TPM multimodal neuroimaging platform at ultrahigh magnetic field, which is expected to reveal the cellular and micro-vascular mechanisms underlying fMRI BOLD signals and revolutionize our understanding on human brain functions and mental disorders from the perspective of neuroimaging.
Acknowledgements
NIH grants: U01 NS094341, R01 MH111413, MH111447 and NS070839, R24 MH106049 and R24 MH106049 S1, P41 EB015894, P30 NS5076408; and the W.M. Keck Foundation.References
1. Zhang, N., et al., High-resolution fMRI mapping of ocular dominance layers in cat lateral geniculate nucleus. Neuroimage, 2010. 50(4): p. 1456-63.
2. Shi, Z.Y., et al., High spatial correspondence at a columnar level between activation and resting state fMRI signals and local field potentials. Proceedings of the National Academy of Sciences of the United States of America, 2017. 114(20): p. 5253-5258.
3. Yacoub, E., Harel, N. & Ugurbil, K. High-field fMRI unveils orientation columns in humans. Proc Natl Acad Sci U S A. 2008;105(30):10607-12.
4. Kong, L., J. Tang, and M. Cui, In vivo volumetric imaging of biological dynamics in deep tissue via wavefront engineering. Opt Express, 2016. 24(2): p. 1214-21.
5. Cui, M., et al., A proof-of-concept study for developing integrated two-photon microscopic and magnetic resonance imaging modality at ultrahigh field of 16.4 tesla. Sci Rep, 2017. 7(1): p. 2733.
Figures