1011
A novel method for mesoscale connectome mapping: focal infrared neural stimulation in high-field functional MRI1Interdisciplinary Institute of Neuroscience and Technology, Qiushi Academy for Advanced Studies, Zhejiang University, Hangzhou, China, 2MR Collaboration NE Asia, Siemens Healthcare, Shanghai, China
Synopsis
Establishing connection patterns between cortical columns is essential for understanding human brain networks. However, currently, there is no method to systematically map at this sub-millimeter scale. Here, we combined pulsed infrared neural stimulation (INS) with high field fMRI. Applying this method in cat and monkey brains, we found that single site INS stimulation produces reproducible, intensity-dependent activation. Our experiments revealed (1) connections between cortex and subcortical locations, (2) long-range cortico-cortical connections, and (3) local cortical connections. We suggest that INS-fMRI is a new in vivo functional tract tracing technique that can map networks with high spatial resolution.
Introduction
Methods
Experiments were conducted in cats and monkeys. All procedures were in accordance with NIH standards and with approval of Zhejiang University Institutional Animal Care Committee. Animals were anesthetized with a combination of propofol or sufentanil supplemented with isoflurane. Data was acquired in a 7T research system (Siemens Healthcare, Erlangen, Germany) with mainly a vendor-supplied single loop coil (RAPID MR International, Columbus, OH, USA) for RF transmission and signal reception. Laser stimulation was delivered via a 200µm diameter optical fiber through either a 1-mm craniotomy or an MR-compatible optical chamber. Stimuli consisted of a train of laser pulses (250µs pulse, 10-200Hz, wavelength 1875nm, radiant energy 0.1-1.0 J/cm2, 0.5 sec). Neural basis of INS-induced activations was examined using optical imaging and electrophysiological methods. Functional images were obtained with a prototype single-shot and a multi-shot echo planar imaging (EPI) sequence and preprocessed with slice-timing and motion correction. Significant response to INS was calculated by identifying voxels with significant F-test p-values with generalized linear models (GLM) (both fast tent function with 2-4 sec peak and slow modified hemodynamic response function with 10-20 sec peak). Analyses were conducted using AFNI, Unix Shell, R and MATLAB scripts.Results
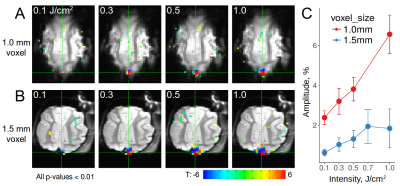
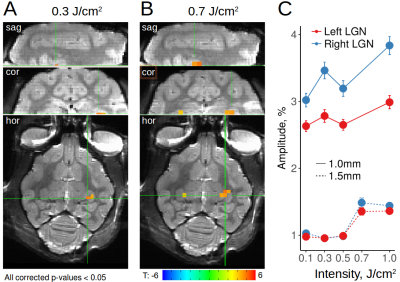
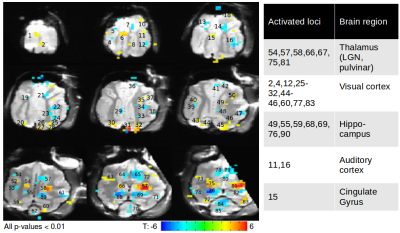
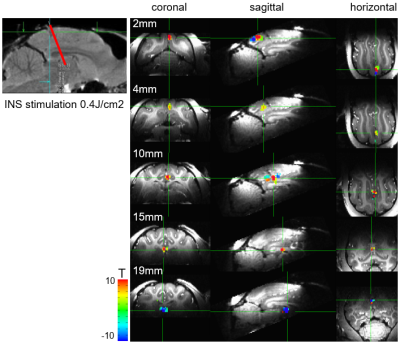
(1) Using radiant energies from 0.1-1.0 J/cm2, we ascertained, with both optical imaging and fMRI, intensity dependence of laser stimulation at the laser tip site (Figure 1). Increasing intensities produced increasing BOLD responses spanning up to several voxels, and was observed for both positive and negative BOLD voxels. We found response magnitude was roughly 3 times lower with 1.5mm voxel size vs 1.0mm voxel size, suggesting a partial volume effect (Figure 1C). (2) Laser stimulation also produced activation at other sites. These included local cortical sites, distant cortical sites, and subcortical sites. For example, stimulation delivered to the right V1 at 0.3 J/cm2 produced activation in right lateral geniculate nucleus (LGN, Figure 2A) and contra-lateral V1, consistent with known direct connections. Stimulation at 0.7 J/cm2 intensity revealed additional activation in left LGN (Figure 2B), consistent with a di-synaptic activation (right V1-left V1-left LGN), raising the possibility of studying mono- vs poly-synaptic activation by controlling laser intensity. For direct connections, LGN activations were intensity dependent and stronger in right (direct) than left (indirect) LGN (Figure 2C). Stimulation in the right V1 also led to activation in multiple visual as well as other cortical and subcortical loci (Figure 3). (3) Using local surface coils, multi-shot sequence, and small voxel sizes, we revealed local intra- and inter-areal connections, consistent with known anatomical connectivity. (4) By scanning cortical laminae at high spatial resolution, INS stimulation revealed activations in adjacent cortical sites confined to the middle layers of cortex, consistent with feedforward connection patterns. (5) Insertion of the optic fiber tip to different depths in the brain produced clear focal activation at each depth (Figure 4).Discussion
Our results show that single site INS stimulation produces reproducible, intensity-dependent activation. Focal INS stimulation reveals (1) connections between cortex and subcortical locations, (2) long-range cortico-cortical connections, and (3) local cortical connections. Our results also suggest potential for mapping direct and indirect connections, and distinguishing between feedforward vs feedback connection patterns. We suggest that INS-fMRI is a new in vivo functional tract tracing technique that can map networks with high spatial resolution.Conclusion
The INS-fMRI technique will have several impacts. By targeting the fiber optic to known functional sites, high spatial resolution functional networks can be quickly revealed. Largescale application promises to lead to a columnar connectome, one in which direct vs indirect connections and feedforward vs feedback connections can be distinguished. This method is also compatible with other imaging, electrophysiological, and behavioral methods for multimodal dataset correlation. The ability to stimulate in deep tissue offers a potentially new approach for therapeutic efforts.Acknowledgements
We would like to thank Lawrence Wald, Jonathan Polimeni, Afonso DeSilva, Wim Vanduffel, and Nick Chernov for helpful discussions. This work was supported by Natual Science Foundations of China (81430010) and the National High Technology Research and Development Program of China (2015AA020515).References
- Cayce J, Friedman RM, Jansen D, Mahavaden-Jansen A, Roe AW (2011) Pulsed infrared light alters neural activity in rat somatosensory cortex in vivo. Neuroimage, 57(1):155-66.
- Cayce J, Friedman RM, Jansen D, Mahadevan-Jansen A, Roe AW (2014) Infrared neural stimulation of primary visual cortex in non-human primates. Neuroimage, 84:181-190.
- Chernov M, Roe AW (2014) Histological assessment of thermal damage in the brain following infrared neural stimulation. Brain Stimulation, 7(3): 476–482.
- Chernov M, Roe AW (2014) Infrared neural stimulation: a new stimulation tool for CNS applications. Neurophotonics, 1(1):011011.
- Izzo AD, Suh E, Pathria J, Walsh JT, Whitlon DS, Richter CP (2007) Selectivity of neural stimulation in the auditory system: a comparison of optic and electric stimuli. J Biomed Opt 12(2):021008.
- Roe AW, Chernov M, Friedman RM, Chen G (2015) In vivo mapping of cortical columnar networks in the monkey with focal electrical and optical stimulation and imaging. Frontiers in Neuroanatomy 9:135.
- Shapiro MG, Homma K, Villarreal S, Richter CP, Bezanilla F (2012) Infrared light excites cells by changing their electrical capacitance. Nat Commun 3:736.
- Wells J, Kao C, Mariappan K, Albea J, Jansen ED, Konrad P, et al. (2005) Optical stimulation of neural tissue in vivo. Opt Lett 30(5):504-6.
- Wells J, Kao C, Jansen ED, Konrad P, Mahadevan-Jansen A (2005) Application of infrared light for in vivo neural stimulation. J Biomed Optics Nov-Dec;10(6).
Figures