1009
Does ventral hippocampus influence auditory processing? An optogenetic fMRI study1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China
Synopsis
Hippocampus has traditionally been associated
Purpose
The hippocampus is an essential brain structure that receives extensive projections from cortices and sensory regions1,2. It is widely believed that hippocampus mediates numerous cognitive functions. For example, the dorsal hippocampus (dHP) plays a role in memory and spatial navigation, while the ventral hippocampus (vHP) is associated with reward-related circuitry and emotional behavior3,4. Our recent study shows that low frequency optogenetic stimulation of the hippocampus enhances bilateral functional connectivity in the sensory cortices such as the auditory cortex5. Meanwhile, a recent study proposed that the hippocampus may be related to auditory memory and sound frequency discrimination6. Despite these findings, it remains unclear whether vHP contributes to the processing of auditory information. Here we combine hippocampal optogenetic stimulation and auditory fMRI to investigate whether vHP influences auditory processing across the entire auditory pathway, including inferior colliculus (IC), medial geniculate body (MGB) and primary auditory cortex (A1).Methods
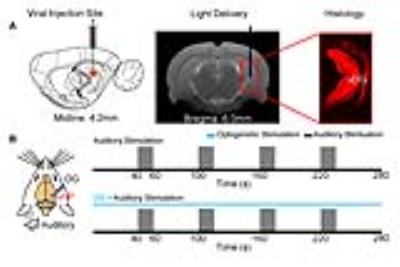
Animal preparation (N=6): 3μl AAV5-CaMKIIα::ChR2(H134R)-mCherry was injected to the ventral dentate gyrus (vDG) in vHP of adult SD rats (200-250g, male). After 4 weeks, an opaque optical fiber cannula (d=450μm) was implanted at the injection site as a means to deliver optical stimulation (Figure 1A). All experiments were performed under 1.0–1.5% isoflurane.
Optogenetic and Auditory Stimulation: To map out the responses of evoked HP optogenetic stimulation, blue light was presented at 5 Hz (30% duty cycle; 40mW/mm2) in a block design paradigm (20s light-on, 140s light-off). To investigate the effects of optogenetic stimulation of vHP on auditory processing, a combined auditory and optogenetic stimulation approach was employed (Figure 1B). Auditory stimulus was delivered via a customized tube to the left ear. Two different sounds were presented (broadband noise: 1-40kHz, 85dB and aversive vocalization: 22kHz, 73dB) in a block design paradigm (20s sound-on, 40s sound-off). Auditory fMRI trials with and without continuous 5Hz optogenetic stimulation were interleaved.
fMRI Acquisition and Analysis: fMRI data was acquired at 7T using GE-EPI (FOV=32×32mm2, matrix=64×64, α=56°, TE/TR=20/1000ms, and 16 contiguous slices with 1mm thickness). Standard fMRI preprocessing was performed before GLM analysis was applied to identify significant BOLD responses (p<0.001). The p-values were calculated by paired t-test for averaged β comparisons between groups.
Results
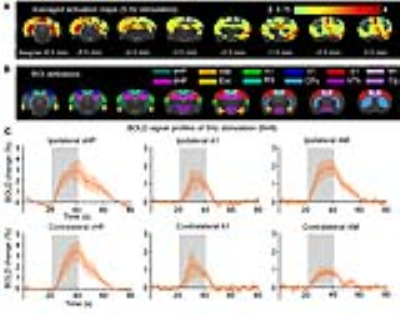
Brain-wide BOLD responses upon 5Hz optogenetic stimulation of vDG excitatory neurons in vHP (Figure 2): Robust positive BOLD responses were detected and characterized in vHP, A1 and the amygdala (AM). These regions were implicated in influencing auditory processing7. Additionally, we also observed activations in other cortical (visual, sensorimotor, cingulate and retrosplenial), hippocampal formation (dHP and entorhinal cortex) and subcortical (caudate putamen and hypothalamus) regions.
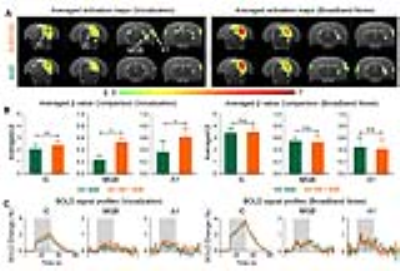
vDG optogenetic stimulation influence auditory processing of behaviorally relevant sound at auditory midbrain, thalamus and cortex (Figure 3): As expected, both broadband noise and aversive vocalization sound evoked robust positive BOLD responses in IC, MGB and A18,9. Interestingly, when optogenetic stimulation was presented with the auditory stimulus, we found that only responses to the vocalization sound were enhanced, but not broadband noise. Such enhancement was found across the auditory pathway such as IC (p<0.01), MGB (p<0.05) and A1 (p<0.05). These findings demonstrate the specificity of the vHP in influencing the processing of specific sounds along the auditory pathway, specifically those that are behaviorally relevant.
Discussions & Conclusion
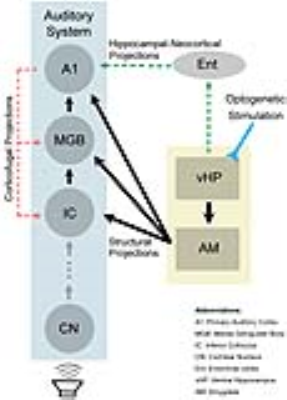
The present study demonstrated the role of hippocampal modulations in auditory processing. We showed that 5Hz optogenetic stimulation of ventral hippocampus (vHP) evoked positive BOLD response in vHP, amygdala and A1. It was found that responses to aversive vocalization, not broadband noise, were significantly increased in the auditory midbrain, thalamus and cortex. This suggests that the hippocampus may be involved in discriminating the characteristics of different auditory stimuli. It has been previously reported that the brain perceives behaviorally relevant sound and simple synthetic sound differently10. The 22kHz vocalization used in the present study is fear and anxiety related11. Sharing reciprocal connections with basolateral amygdala (BLA)12, vHP has been shown to interact with BLA to mediate anxiety-related behavior13. Furthermore, there are direct neuronal projections from BLA to IC, MGB and AC14-16. Taken together, the interactions between vHP and amygdala could influence vocalization processing in IC, MGB and A1 (Figure 4). Alternatively, processing of vocalization sound in A1 may also be affected by hippocampal activity through the hippocampal-neocortical network1,17, and MGB and IC via the corticofugal projections8. In summary, our study presents valuable insights into the role of the ventral hippocampal activity on auditory processing. We present for the first time, direct experimental evidence that the ventral hippocampus influences auditory processing of behaviorally relevant sound at auditory midbrain, thalamus and cortex.Acknowledgements
This work was supported by the Hong Kong Research Grant Council (Grants C7048-16G and HKU17103015 to E.X.W.).References
1. Buzsáki, G. (1996). The hippocampo-neocortical dialogue. Cerebral cortex, 6(2), 81-92.
2. Van Strien, N. M., Cappaert, N. L. M., & Witter, M. P. (2009). The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nature Reviews Neuroscience, 10(4), 272-282.
3. Fanselow, M. S., & Dong, H. W. (2010). Are the dorsal and ventral hippocampus functionally distinct structures?. Neuron, 65(1), 7-19.
4. Strange, B. A., Witter, M. P., Lein, E. S., & Moser, E. I. (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience, 15(10), 655-669.
5. Chan, R.W., Leong, A.T., Ho, L.C., Gao, P.P., Wong, E.C., Dong, C.M., Wang, X., He, J., Chan, Y.S., Lim, L.W. & Wu, E.X. (2017). Low-frequency hippocampal–cortical activity drives brain-wide resting-state functional MRI connectivity. Proceedings of the National Academy of Sciences, 114(33), E6972-E6981.
6. Aronov, D., Nevers, R., & Tank, D. W. (2017). Mapping of a non-spatial dimension by the hippocampal–entorhinal circuit. Nature, 543(7647), 719-722.
7. Kraus, K. S., & Canlon, B. (2012). Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hearing research, 288(1), 34-46.
8. Gao, P. P., Zhang, J. W., Fan, S. J., Sanes, D. H., & Wu, E. X. (2015). Auditory midbrain processing is differentially modulated by auditory and visual cortices: An auditory fMRI study. Neuroimage, 123, 22-32.
9. Zhang, J. W., Lau, C., Cheng, J. S., Xing, K. K., Zhou, I. Y., Cheung, M. M., & Wu, E. X. (2013). Functional magnetic resonance imaging of sound pressure level encoding in the rat central auditory system. Neuroimage, 65, 119-126.
10. Theunissen, F. E., & Elie, J. E. (2014). Neural processing of natural sounds. Nature Reviews Neuroscience, 15(6), 355-366.
11. Portfors, C. V. (2007). Types and functions of ultrasonic vocalizations in laboratory rats and mice. Journal of the American Association for Laboratory Animal Science, 46(1), 28-34.
12. Felix-Ortiz, A. C., Beyeler, A., Seo, C., Leppla, C. A., Wildes, C. P., & Tye, K. M. (2013). BLA to vHPC inputs modulate anxiety-related behaviors. Neuron, 79(4), 658-664.
13. Felix-Ortiz, A. C., & Tye, K. M. (2014). Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. Journal of Neuroscience, 34(2), 586-595.
14. Marsh, R. A., Fuzessery, Z. M., Grose, C. D., & Wenstrup, J. J. (2002). Projection to the inferior colliculus from the basal nucleus of the amygdala. Journal of Neuroscience, 22(23), 10449-10460.
15. Poremba, A., & Gabriel, M. (2001). Amygdalar efferents initiate auditory thalamic discriminative training-induced neuronal activity. Journal of Neuroscience, 21(1), 270-278.
16. Janak, P. H., & Tye, K. M. (2015). From circuits to behaviour in the amygdala. Nature, 517(7534), 284-292.
17. Sirota, A., Montgomery, S., Fujisawa, S., Isomura, Y., Zugaro, M., & Buzsáki, G. (2008). Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron, 60(4), 683-697.
Figures