1005
3D ultrasound based motion tracking combined with rapid multislice MR-thermometry for MR guided HIFU on mobile organs.1IHU-LIRYC, PESSAC, France, 2Univ. Bordeaux, Centre de recherche Cardio-Thoracique de Bordeaux, Bordeaux, France, 3INSERM U1045, Bordeaux, France, 4Image Guided Therapy, Pessac, France, 5Institute of Mathematics of Bordeaux, Bordeaux, France
Synopsis
MRI-guided High Intensity Focused Ultrasound treatment of mobile organs remain challenging in terms of therapy effectiveness (target tracking) and precision of temperature monitoring (motion and related susceptibility artifacts). In this study, we propose to use some elements of the HIFU transducer in reception for a standalone motion correction of the focus position, allowing full flexibility on the MR-Thermometry sequence. The method is validated in vitro on a mobile gel and in vivo in the liver of pig during breathing, with a 10Hz update of the HIFU focus position under rapid and volumetric MR-thermometry.
Introduction
MRI-guided High Intensity Focused Ultrasound (MR-HIFU) treatment of mobile organs (heart, liver, kidney) remains challenging since it requires compensation of motion on MRI thermometry and real-time update of HIFU beam position to track the targeted region. As of now, motion corrections in MRI have been proposed using a MRI echo navigator [1], image registration [2] or using external sensors [3]. However, the aforementioned methods either slave the motion correction on MR-acquisition or lack in 3D correction. To overcome these limitations, we proposed to use a 3D, ultrasound-based motion correction [4] using some elements of the HIFU probe in transmit-receive mode. This method does not require any additional device inside the magnet and do not require compromises on MR-thermometry. A validation was performed on agar gel under controlled motion and a proof in vivo in the liver of pig.
Materials & Methods
3D motion tracking was implemented using ultrasonic speckle-tracking on four sub-apertures of the HIFU transducer . Estimation of the displacement was performed at 10 Hz update rate, based on the cross-correlation of backscattered signals between consecutive HIFU shots of 2 ms duration followed by a triangulation algorithm (see Figure 1).
$$T_i=\frac{2}{c}A_iD$$
Where $$$T_i$$$ is the temporal lag between successive acquisitions matrix, $$$A_i$$$ is the normalized 3D sub-apertures matrix, c is the speed of sound and $$$D$$$ is the 3D displacement matrix. The computed displacement was exploited in real-time to steer the HIFU beam to track the target. MR-temperature imaging (proton resonance frequency shift method) was preformed simultaneously using a fast echo planar imaging sequence, as previously published [5]. During in vitro experiments, three slices were acquired in coronal orientation with the following parameters: FOV = 180x168 mm², TR/TE/FA = 166 ms/14 ms/32 °, voxel size = 2x2x5 mm$$$^3$$$, partial Fourier 6/8, with a bandwidth of 1650 Hz per pixel. For in vivo experiments in the liver of pig (see Figure 1), five slices were acquired: FOV = 224x224 mm², TR/TE/FA = 140 ms/14 ms/45 °, voxel size = 2x2x5 mm$$$^3$$$, partial Fourier 6/8, with a bandwidth of 1860 Hz per pixel. The method was evaluated on an agar gel positioned on a mobile platform allowing an oscillating translation in the head-feet direction with a controlled amplitude and frequency. Then, the method was evaluated in vivo in the liver of two pigs under general anaesthesia and assisted ventilation.
Results
On an Agar gel (Figure 2), displacement estimations on y axis were in agreement with a 1D navigator echo readings, positioned in the head-feet direction (amplitude error < 1%). The maximum displacement and velocity measured were 12 mm and 11 mm.s-1, respectively. Ablation was performed with a sonication duty cycle of 63 % during 60 s at 150 W acoustic power. The maximum temperature increase was 18±1.2 °C and 26±1.2 °C (Figure 3) without and with focus correction, respectively. On the same slice, the peak width at half maximum of the temperature distribution along y axis, at the end of the sonication were 18 mm and 6 mm without and with correction, respectively. Displacement estimations in vivo on y axis, were in good agreement with the echo navigator signal positioned at the liver-lung interface (Figure 4). The maximum displacement and velocity measured were 10 mm and 11 mm.s-1, respectively. Ablation was performed in vivo during 40 s at 450 W acoustic power. The maximum temperature increase was 18±0.9 °C and 27.0±0.9 °C (Figure 5) without and with focus correction, respectively. The peak width at half maximum of the temperature profiles along the y direction were 12 and 8 mm without and with correction, respectively.Discussions and conclusions
Motion correction and online HIFU beam steering were successfully
performed in vitro and in vivo with a 10Hz update in position. In vitro and in
vivo, efficient target locking was confirmed by temperature evolution and
displayed a complete compensation on the motion artifact observed when motion
correction was disabled. After focus position correction the heating
effectiveness was improved by approximately 35 % in vitro and in vivo. Further hardware
and software optimization may provide higher frame rate for 3D motion
determination and compensation.Acknowledgements
This work received financial support from the French National Investments for the Future Programs: ANR-10-IAHU-04 (IHU Liryc) and Laboratory of Excellence ANR-10-LABX-57 (TRAIL), and the research programs ANR-11-TecSan-003-01 (TACIT) and Equipex ANR-11-EQPX-0030 (MUSIC). Authors gratefully acknowledge Valentine Prat for her assistance in animal experimentation.References
[1] Z. Celicanin et al., « Real-time method for motion-compensated MR thermometry and MRgHIFU treatment in abdominal organs: MRgHIFU Treatment Method in Abdominal Organs », Magn. Reson. Med., vol. 72, no 4, p. 1087‑1095, oct. 2014.
[2] B. D. de Senneville, M. Ries, G. Maclair, et C. Moonen, « MR-Guided Thermotherapy of Abdominal Organs Using a Robust PCA-Based Motion Descriptor », IEEE Trans. Med. Imaging, vol. 30, no 11, p. 1987‑1995, nov. 2011.
[3] Z. Celicanin et al., « Hybrid ultrasound-MR guided HIFU treatment method with 3D motion compensation », Magn. Reson. Med., sept. 2017.
[4] M. Pernot, M. Tanter, et M. Fink, « 3-D real-time motion correction in high-intensity focused ultrasound therapy », Ultrasound Med. Biol., vol. 30, no 9, p. 1239‑1249, sept. 2004.
[5] V. Ozenne et al., « Improved cardiac magnetic resonance thermometry and dosimetry for monitoring lesion formation during catheter ablation », Magn. Reson. Med., p. n/a-n/a, févr. 2016.
Figures
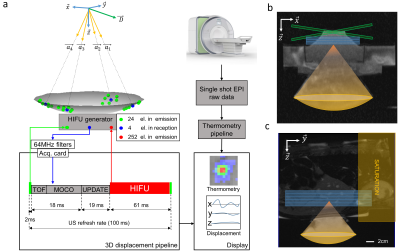
Figure 1: a: Schematic of the 3D ultrasound-based motion estimation system. Transducer elements operating in emission are represented in green and those in reception in blue, respectively. The pipeline of 3D displacement estimation is presented underneath. TOF: Time Of Flight for ultrasound to reach the depth of interest; motion correction (MOCO): computing time for the displacement estimation, UPDATE: transducer phase law update.
b: Transverse anatomical image of the setup. Blue box represent three slices in coronal orientation and green box represent the crossed pair navigator position.
c: Anatomical image and animal positioning. Blue box represent five MR-temperature slices positioned in coronal orientation.
Figure 2: Displacement estimation validation in vitro.
a: MR-navigator signal positioned along the y-axis acquired during 21 s.
b: Representative displacement estimations in (x,y,z) obtained in vitro using our experimental settings. The blue and green curves represent the displacement estimations and the corresponding instantaneous speed computed from estimated positions, respectively. The bottom graph displays the cross-correlations between two successive US acquisitions along time, for each sub-apertures. Black arrows depict lower cross-correlation coefficients during peak velocities.
Figure 3: Representative in vitro MR-Thermometry.
a: Shows temperature images overlaid to magnitude images, taken at the end of HIFU sonication, without (top) and with (bottom) 3D focus position update.
b: Shows the temperature temporal evolution of the pixel experiencing the maximum temperature increase, for both conditions. The 50 first dynamic acquisitions (gray box) corresponded to the learning step in the thermometry pipeline and were used for correction of motion and associated susceptibility artifacts of temperature images.
c: shows the temperature profiles along the y-axis crossing the pixel with maximal temperature increase, at the end of sonication, for both conditions.
Figure 4: Displacement estimation in vivo in pig liver.
a: MR-navigator signal positioned along the y-axis acquired during 30 s.
b: Representative displacement estimations in (x,y,z) obtained during in vivo experiment on liver. The blue and green curves represent the displacement estimations and the corresponding instantaneous speed computed from estimated positions, respectively. The bottom graph displays the cross-correlations between two successive US acquisitions along time, for each sub-apertures. The red curve displays the mean correlation between current and the first acquisition taken as reference. The black dashed line represents the threshold above which absolute displacement estimations is performed to reset the position.
Figure 5: Representative in vivo MR-Thermometry.
a: Screen capture of representative temperature images overlaid to magnitude image, taken at the end of HIFU sonication, without (top) and with (bottom) focus update.
b: Shows the temperature evolution of the pixel experiencing the maximum temperature increase, along time, for both conditions. The 50 first dynamic acquisitions (gray box) corresponded to the learning step in the thermometry pipeline (see material and methods for details).
c: shows the temperature profile along the axis crossing the pixel with maximal temperature increase at the end of sonication, for both conditions.